Abstract
Abstract 1947
Sclerotic cGvHD is one of the most severe complications after allogeneic hematopoietic stem cell transplantation (HSCT). The sclerotic skin manifestations of cGvHD result from inflammation and fibrosis of the dermis, subcutaneous tissue, or muscular fascia, leading to functional disability. Risk factors associated with sclerotic cGvHD remain not well studied. TBI, PBSC, female recipient, and prior severe skin acute GvHD were identified as factors associated with an increased risk of sclerotic cGvHD in a large previous retrospective study from Seattle (n=986 patients) which was presented at the ASH 2011 meeting.
In the aim to validate the previous risk factors in France, we have conducted this retrospective multicentric analysis. We present here the preliminary results from four centers, seven are expected in the next months. Between 2005 and 2010, 437 consecutive patients who received systemic therapy for chronic GvHD after a first allogeneic HSCT were included. Chronic GvHD was diagnosed by the NIH consensus criteria. Sclerotic GvHD was defined by manifestations of cutaneous sclerosis, fasciitis or joint contracture in the medical record. Analyses to determine risk factors included as variable patient and donor age, kind of donor, HLA matching, diagnosis, stem cell source, patient and donor gender, intensity of conditioning regimen, use of total body irradiation (TBI) or antithymocyte globulin (ATG) in the conditioning regimen, and prior acute GvHD.
All patients had hematological malignancies, the median age is 45 (17–65), 227 patients (52%) had HLA-matched related donors, 125 (29%) had HLA-matched unrelated donors, 48 (11%) had HLA- 9/10 unrelated donors and 37 (8%) had one or two cord blood unit (HLA-4/6 or 5/6 matched). The median time from allogeneic HSCT to chronic GvHD was 6.5 (1.8–41.5) months; 69 patients (15.7%) presented sclerotic features at initial diagnosis or after the onset of chronic GvHD.
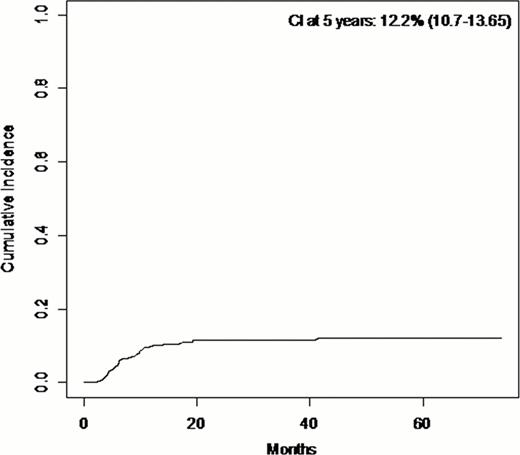
The cumulative incidence of sclerotic cGvHD was 12% (95%CI, 11–13.5) at 5 years after onset of chronic GvHD (Figure). From the 69 patients who experienced sclerotic cGvHD, 49 patients (71%) had HLA-matched related donors, 19 patients (27.5%) had HLA-matched unrelated donors, and 1 patient (1.5%) had HLA-mismatched unrelated donor. In univariate analysis, we found more sclerotic cGvHD in patients who had multiple myeloma (p=0.018); this effect seems to be associated with the prophylaxis for GvHD with CsA alone. There was no effect of TBI or intensity of conditioning. There was more sclerotic GvHD in female recipient (p=0.032) and for patients who had an unrelated donor (p=0.0028).
In multivariate analysis (Table), factors associated with an increased risk of sclerotic cGvHD were female recipient, patients with unrelated donor, and patients with previous aGvHD. Cord blood as stem cell source was associated with a lower risk of sclerotic GvHD. Prophylaxis of GvHD with CsA alone showed a trend to a higher risk. At the last follow-up, of the 69 patients, 51 (74%) were alive and 10 (14.5%) died from cGvHD.
In this intermediate analysis, we found that female recipient, unrelated donor, previous aGvHD are risk factors for developing sclerotic cGvHD. Moreover, we found that cord blood as stem cell source is a protector factor. Study of GvHD prophylaxis, ABO matching, type of HLA-mismatching, acute skin GvHD is ongoing on the whole cohort (n= 1587 patients from 7 centers) and results will be communicated later.
After the final analysis on the whole cohort we would like to establish a score risk of sclerotic cGvHD in the goal to conduct a prospective study in France with a prophylactic treatment for high risk patients.
Multivariate analysis
| . | HR . | p . |
|---|---|---|
| Stem cells source and conditioning intensity | ||
| MAC + Bone marrow | 1.0 | |
| RIC + Bone marrow | 2.03 (0.39–10.6) | .4 |
| MAC + Cord blood | 0.0 | .00001 |
| RIC + Cord blood | 0.68 (0.02–18.01) | .82 |
| MAC + PBSC | 0.32 (0.081–1.28) | .11 |
| RIC + PBSC | 1.15 (0.5–2.5) | .73 |
| HLA and Donor | ||
| Matched related | 1.0 | |
| Matched unrelated | 0.04 (0.011–0.181) | .00001 |
| Mismatched unrelated | 0.62 (0.13–2.7) | .53 |
| Patient age | NS | |
| Donor age | NS | |
| Sexmatching | ||
| Male to male | 1.0 | |
| Female to female | 2.7 (1.2–6.06) | .016 |
| Female to male | 1.8 (0.78–4.33) | .16 |
| Male to female | 1.44 (0.67–3.13) | .34 |
| Disease diagnosis | NS | |
| ATG in conditioning | NS | |
| TBI in conditioning | 1.35 (0.69–2.64) | .38 |
| Prior acute GvHD | 1.8 (0.9–3.36) | .064 |
| . | HR . | p . |
|---|---|---|
| Stem cells source and conditioning intensity | ||
| MAC + Bone marrow | 1.0 | |
| RIC + Bone marrow | 2.03 (0.39–10.6) | .4 |
| MAC + Cord blood | 0.0 | .00001 |
| RIC + Cord blood | 0.68 (0.02–18.01) | .82 |
| MAC + PBSC | 0.32 (0.081–1.28) | .11 |
| RIC + PBSC | 1.15 (0.5–2.5) | .73 |
| HLA and Donor | ||
| Matched related | 1.0 | |
| Matched unrelated | 0.04 (0.011–0.181) | .00001 |
| Mismatched unrelated | 0.62 (0.13–2.7) | .53 |
| Patient age | NS | |
| Donor age | NS | |
| Sexmatching | ||
| Male to male | 1.0 | |
| Female to female | 2.7 (1.2–6.06) | .016 |
| Female to male | 1.8 (0.78–4.33) | .16 |
| Male to female | 1.44 (0.67–3.13) | .34 |
| Disease diagnosis | NS | |
| ATG in conditioning | NS | |
| TBI in conditioning | 1.35 (0.69–2.64) | .38 |
| Prior acute GvHD | 1.8 (0.9–3.36) | .064 |
NS= not statistically significant.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal