Abstract
Abstract 1937
Despite the advantages of autologous stem cell transplantation (ASCT) over conventional chemotherapy, the results of ASCT in multiple myeloma (MM) are still unsatisfactory. Intravenous (iv) melphalan at a dose of 200 mg/m2 is the most commonly used conditioning regimen for ASCT. However, better conditioning regimens are still needed, and more intensive conditioning regimens have been investigated. We evaluated the role of bortezomib-containing regimen (escalated dose of bortezomib + iv busulfan and iv melphalan) in the MM patients who had a sensitive response to bortezomib during the induction chemotherapy. In the phase 1 trial, we assessed the MTD (maximum tolerated dose) of bortezomib. The trial is registered on National Cancer Institute website, number NCT01255527.
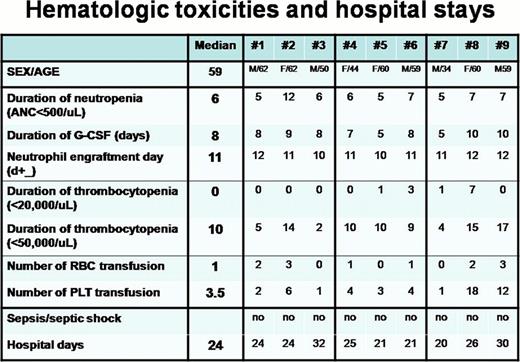
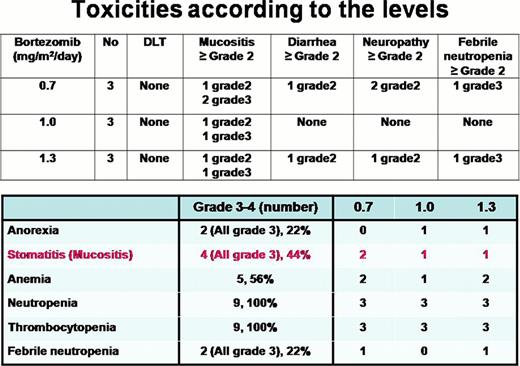
Nine MM patients who were candidate for ASCT enrolled in this study. M:F=5:4 and median age were 59 (34–62). They were treated with escalating doses of bortezomib as a conditioning regimen (N=3/group; 0.7, 1.0, and 1.3 mg/m2 on D-6, D-3 & D+1) and a fixed dose of busulfan (3.2 mg/kg from D-5 to D-3) and melphalan (140 mg/m2 on D-2). Dose limiting toxicities (DLT) were defined as any toxicity of grade 3 or greater, with the following exceptions: vomiting, fatigue, alopecia, libido, amenorrhea, nausea, febrile neutropenia, infection, anorexia, depression, anxiety, and cytopenias. Grade 3/4 hematological toxicity was acceptable. Grade 4 of mucositis, °Ã Grade 3 diarrhea and °Ã Grade 3 cardiac toxicity were regarded as DLT. Only one patients had grade 3 oral mucositis and there were no other non hematologic toxicities of grade 3 or greater. DLT refers only to toxic events (symptomatic grade °Ã 3 toxicity) that occur until day +28 after ASCT and are attributed as possibly, probably, or definitely due to treatment.
Four patients had grade 3 oral mucositis (2 in 0.7 mg/m2 group, 1 in 1.0 mg/m2 group and 1 in 1.3 mg/m2 group), but no grade 4 (considered as DLT) oral mucositis. No other non hematologic toxicities of grade 3 or greater. Therefore, there were no dose limiting toxicities in this phase 1 trial and MTD of bortezomib was 1.3 mg/m2. Median times to ANC > 0.5 · 106/L and platelet > 20 · 106/L were 12 and 10 days, respectively, with no graft failures. All patients achieved at least very good partial response. Six patients (67%) achieved complete remission(CR) after ASCT.
In the phase 1 trial, MTD of bortezomib was 1.3 mg/m2. Addition of bortezomib to busulfan and melphalan conditioning regimen shows to be safe, well-tolerated and worthy of phase 2 trial.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal