Abstract
Abstract 193
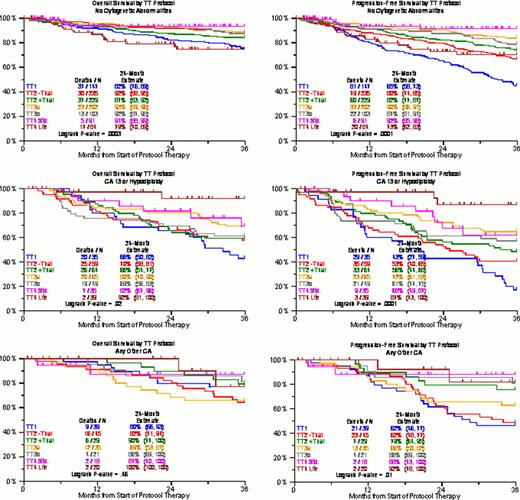
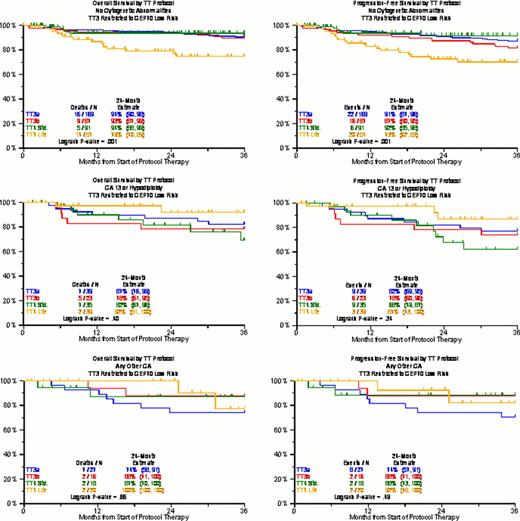
We have previously reported on the adverse prognostic implications of CA, independently contributing to clinical outcome prediction even in the era of gene expression profiling (GEP). The presence of hypodiploidy/deletion13 (CA13/hypo) was associated with a particular poor prognosis, whereas the absence of CA (no CA) imparted superior outcome; the remainder (other CA) had an intermediate prognosis. Here we provide an update of overall survival (OS) and progression-free survival (PFS) with TT1, TT2 control arm (TT2-), TT2 thalidomide arm (TT2+), TT3a, TT3b, TT4-light (TT4-L, fractionated melphalan + VTD), and TT4-standard (TT4-S). The backbone of protocols was tandem transplantation with melphalan 200mg/m2 (MEL-200). TT2 employed thalidomide during all phases of the protocol in a randomized fashion. TT3a introduced bortezomib and 3-yr maintenance with VTD for year 1 and TD for years 2 and 3; TT3b applied VRD with lenalidomide in all 3 years of maintenance; TT4-S used standard MEL-200 as conditioning, whilst in TT4 MEL-50 for 4 consecutive days was given. In addition, TT4L applied 1 instead of 2 cycles of induction and consolidation therapy. Both OS and PFS were consistently superior with “no CA” followed by “other CA” and “CA13/hypo” in all trials including TT4-S (Figure 1). However, there was a striking reversal of the benefit order with TT4-L: “no CA” fared worst, and both “CA13/hypo” and “other CA” had equally superior OS and PFS. The analyses were repeated for TT3 and TT4, limiting TT3 to the ∼85% of patients with GEP-defined low-risk MM, since TT4 was designed specifically for GEP70 low risk MM (Figure 2). In the “no CA” group, 2-yr OS and PFS were >90% in TT3a/b and TT4-S, compared to 75% in TT4-L (p=0.007 and 0.01 for TT3 and TT4-s, respectively). TT4-L patients had ∼90% 2-yr OS and PFS in case of “CA13/hypo” and “other CA”, contrasting with ∼60–80% 2-yr OS and PFS for the other protocols. It could be speculated that patients with “no CA” have more slowly cycling micro-environment dependent disease in which the well-recognized MEL dose response relationship is crucial. Conversely, the more genomically unstable “CA13/hypo”-type MM may benefit from the synergistic effect of repeated application of MEL and bortezomib, which could be related to the ability of bortezomib to disrupt protein metabolism required for repair of MEL-induced DNA damage. The basis for these finding is under investigation, but do support the need for further studies investigating novel conditioning regimens and their exact beneficiaries, which could further personalize myeloma care and may be related to bortezomib's presence in the fractionated melphalan transplant regimen in TT4-L.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal