Abstract
Abstract 1914
Bendamustine has demonstrated efficacy as single agent in several lymphoproliferative disorders, including Hodgkin's lymphoma (HL). Despite the wide use of this compound, alone or in combination, there are no published data regarding its mobilizing activity. In 2011, we started a phase II open-label prospective study with Bendamustine, Gemcitabine and Vinorelbine (BeGEV) to evaluate the efficacy of this induction regimen before high dose chemotherapy plus autologus stem cell transplant (ASCT). One of the study objectives was to detect the role of Bendamustine as part of a mobilizing regimen for peripheral blood stem cell (PBSC) collection.
Between August 2011 and July 2012, 16 consecutive patients with relapsed/refractory HL were enrolled in a Phase II open-label prospective study with BeGEV followed by ASCT. The treatment schedule was: Bendamustine (90mg/sqm, days 2–3), Gemcitabine (800mg/sqm, day 1 and 4) and Vinorelbine (25mg/sqm, day 1) plus G-CSF 10mcg/Kg beginning on day 7 continued daily until the target yield would be reached. PBSC collection was planned starting from cycle 1 or from cycle 3 in case of bone marrow involvement. Three million CD34+/Kg were considered as the minimum cell dose established for a safety rescue. Other than successful rate of harvest, we evaluated the absolute number of collected CD34+ cells/Kg, the number of procedures performed per cycle, preleukapheresis circulating CD34+ cells/mcL, white blood cells (WBC) count and the day of first collection. Adverse events were also recorded. All patients provided written informed consent at the time of study inclusion.
Of the 16 patients enrolled, 14 already underwent leukapheresis.
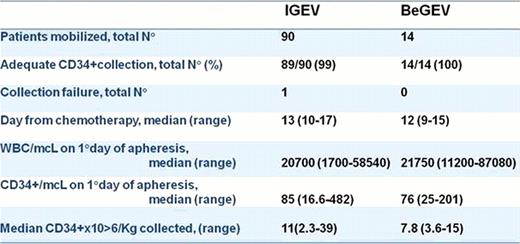
All patients were able to mobilize readily and all achieved the primary end point with at least 3.6 × 10>6 CD34+/Kg collected in a single cycle. The median yield of CD 34+/Kg collected was 7.8 × 10>6 CD34+/Kg (range, 3.6–15) after a median of 1 procedure (range, 1–2). The median preleukapheresis circulating CD34+/mcL and WBC count/mcL were 76/mcL (range, 25–201) and 21750/mcL (range, 11200–87080), respectively. The median day of first collection was 12 (range, 9–15). Six pts underwent leukapheresis at cycle 1, 7 pts at cycle 2 (6 pts due to logistic reasons,1 to Cytomegalovirus reactivation). One pt underwent leukapheresis at cycle 3 for personal reasons obtaining the highest yield (15×10>6 CD34+/Kg). Hematologic and non-hematologic side effects were acceptable and no toxic deaths occurred. One patient developed blood-pressure decrement during the apheresis but she was able to complete the procedure without sequelae. To date, 6 patients (43%) underwent ASCT with prompt engraftment. Data about neutrophils and platelets engraftment will be presented in the final analysis. Comparison with historical IGEV published data (Magagnoli et al, BMT 2007) is reported in Table 1.
This is the first prospective study evaluating Bendamustine as mobilizing agent in resistant HL pts before ASCT. Despite the small sample, our results show that BeGEV regimen, combined with G-CSF support, can be successfully and safely used to mobilize PBSC.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal