Abstract
Abstract 1830
Multiple myeloma (MM) is a universally fatal disease, comprising 15% of hematological malignancies and 1% of all cancers. It has a median survival of 5–7 years from diagnosis. While newer drugs, such as the proteasome inhibitor bortezomib, have increased response to therapy, resistance and relapse still remain a key concern. Therefore, there is a need to understand the mechanism(s) of drug resistance. Ideally, this needs to be accomplished in a patient-specific manner. One approach is to directly examine biological responses of patient myeloma cells in the presence of their own stromal components under different drug conditions. However, individually collected patient bone marrow samples yield a limited number of MM tumor and associated stromal cell types, which limit the number of conditions as well as replicates that can be performed for such functional analyses.
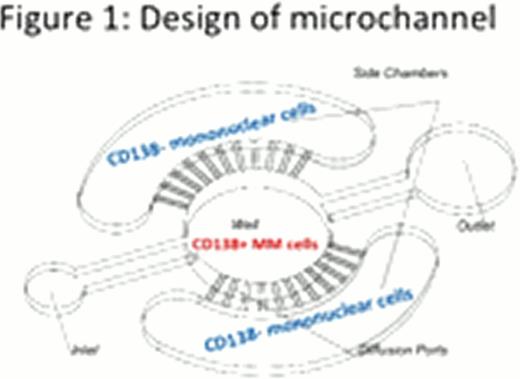
To address this technical barrier, we have developed a first generation functional microfluidics-based microchannel platform that enables the analysis of these limited samples in mono- and/or cis-coculture (Figure 1). CD138+ patient myeloma cells are seeded into the central channel, which has a well to retain the suspension MM cells. CD138- “stromal” or mononuclear cells are seeded into each side channel. Each side channel communicates with the central channel via diffusion ports, which are only large enough for media and soluble factors to flow through. In this manner, both without (monoculture) and with the patients own stromal components (cis-co-culture) can be analyzed for MM cell responses.
Here we employed this assay platform to analyze CD138+ MM tumor cells' drug responses to the proteasome inhibitor bortezomib/Velcade and whether CD138- mononuclear cells from the same patient provide a protective effect. We added 7500 CD138+ in the central channel with or without 8000 CD138- mononuclear cells in each side channel, which were obtained from the same bone marrow aspirates. We were able to measure dose-dependent reductions in cell viability in multiple patient MM cells. Such MM cell viability was protected by the presence of patients' own CD138- mononuclear cells to varying degrees.
More patient samples are currently being analyzed and will be similarly assessed to determine whether such in vitro bortezomib responses have any relevance to patient drug responses in the clinic. Additional improvements will be incorporated with the goal to make the assay platform more robust and quantitative in its predictive capacity.
Beebe:Bellbrook Labs: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal