Abstract
Abstract 1747
The oral JAK 1/2 inhibitor ruxolitinib (RUX) was approved in November 2011 for patients (pts) with IPSS intermediate (INT) or high-risk myelofibrosis (MF). In the COMFORT-I and –II trials, RUX was superior to placebo or best available therapy, respectively, based on its ability to improve splenomegaly and MF-related symptoms (Verstovsek et al; Harrison et al, New Engl J Med., 2012). Eligibility for both trials required a platelet count at study entry of >100 × 109/L. The initial RUX dose was based on the starting platelet count (100–200 × 109/L: 15 mg BID; >200 × 109/L: 20 mg BID), with on-trial dosing optimized for efficacy and safety.
In our single-center experience, we initiated RUX treatment in 23 pts (15 men) from December 2011 through June 2012. Baseline characteristics of the cohort included the following: median age 72 years (range 55–83); MF subtypes: 15 PMF, 7 post-PV MF, 1 post-ET MF; DIPSS (PLUS) risk groups: INT-1 (n=2), INT-2 (n=14), and high risk (n=7); mutation status: 18 JAK2 V617F (V617F) positive, 3 wild-type JAK2, and 2 pts with a MPL 515 mutation; mean baseline palpable splenomegaly 17 cm (4–26 cm). The median # prior therapies was 1 (range 0–6), including 4 pts on prior JAK inhibitors. One pt was discontinued from RUX therapy on the COMFORT-I trial for worsening splenomegaly / symptoms related to protocol-specified dose reduction to 5 mg BID for thrombocytopenia. RUX was dosed similar to the COMFORT trials, with 10 mg BID administered to 5 of 6 pts with a platelet count < 100 × 109/L (the 1 pt on the COMFORT-1 trial was dosed at 15 mg BID). International Working Group (IWG) criteria were used to evaluate clinical benefit.
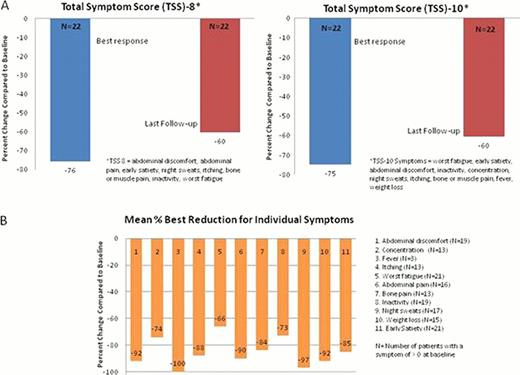
The median duration of therapy was 20 weeks (wks) (range 3–31). The mean total daily dose exposure was 32 mg (range 9–47 mg). Efficacy: Among 20 pts evaluable for reduction of palpable splenomegaly (>8 wks on RUX and no prior splenectomy), the rate of IWG-defined clinical improvement (CI; >50% decrease maintained for >8 wks) was 15%. A > 50% reduction in splenomegaly at any point in time was observed in 40% of pts. The median absolute and % maximal reductions in splenomegaly were 6 cm (range 2–11 cm) and 40%. The proportion of pts achieving >75%, 51–75%, 25–50%, and <25% maximal reduction in splenomegaly was 1/20 (5%), 7/20 (35%), 9/20 (45%), and 3/20 (15%), respectively. In pts with baseline splenomegaly of <10 cm (n=5) the mean absolute and % maximal reductions were 4 cm (range 2–6 cm) and 63%; in pts with baseline splenomegaly >10 cm (n=15), mean absolute and % maximal reductions were 7 cm (range 4–11 cm) and 38%. Two of the 6 pts (33%) with a baseline platelet count < 100 × 109/L achieved a CI for splenomegaly. The mean best weight gain on RUX was 4.3 kg (range 0.5 to 11.7 kg). A >50% reduction in the 8- and 10-point total symptom scores (TSS), derived from the MPN symptom assessment form, was observed in 96% and 91% of pts, respectively. The mean % best reductions in TSS-8 and TSS-10 are shown in the Figure (Panel A), and the mean % best reduction in individual symptoms is shown in Panel B. Among 16 pts with a median follow-up of 12 wks (range 8–28 wks), the mean change in V617F allele burden (%) was −1.5% (range −26% to 20%). Safety: All 23 pts were evaluable for safety. NCI CTC v4.0 was used to grade adverse events (AEs). Overall, RUX was well tolerated. Higher grade non-hematologic AEs considered drug-related included one pt with grade 3 increased AST/ALT and one pt with grade 2 increased total bilirubin (also in the setting of ethanol abuse, leading to RUX discontinuation after 3 wks). Four pts underwent RUX dose reduction (grade 3 AST/ALT; grade 3 thrombocytopenia; grade 2 thrombocytopenia [platelet count 50,000 with epistaxis]; and worsening of a pre-existing red blood cell (RBC) transfusion requirement, each n=1). Five of 23 pts (22%) (or 5/15 pts (33%) initially transfusion independent) developed a new RBC transfusion-requirement /grade 3 anemia, defined as >2 units in any 8-wk period on RUX. Similarly, 5/23 pts (22%) had emergent grade 3 thrombocytopenia (baseline platelet counts 58, 73, 88, 96, and 483 × 109/L).
In our cohort, the efficacy and safety results obtained with commercial ruxolitinib are generally similar to prior trials. Some observed differences (e.g. lower CI rate for splenomegaly) may be related to baseline pt characteristics (e.g. inclusion of pts treated with prior JAK inhibitors) and the need for longer observation.
Gotlib:Incyte Corporation: Consultancy, Honoraria, Support for travel to meeting for the study or other purposes from Incyte Other.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal