Abstract
The opportunity to discontinue kinase inhibitor therapy while maintaining a deep remission is desirable for many CML patients. Despite good responses to imatinib for most patients, treatment related side effects remain problematic and can affect quality of life. Studies have demonstrated that a proportion of carefully selected patients can sustain response after imatinib discontinuation. The first requirement for successful discontinuation is likely to be stable deep molecular response based on a sensitive RQ-PCR assay. The criteria for patient selection in the French Stop Imatinib (STIM) and Australian CML8 (TWISTER) imatinib discontinuation trials included stable undetectable BCR-ABL1 transcripts for at least 24 months with a PCR sensitivity of 5 and 4.5 log, respectively. The probability of continued remission after discontinuation for imatinib treated patients without prior interferon-α therapy was approximately 33%. It is not known how many imatinib treated patients will eventually meet these PCR criteria for a discontinuation trial.
We aimed to determine 1) the cumulative probability of achieving the PCR criteria for imatinib discontinuation as defined in the CML8 study, and 2) factors that predicted its achievement.
The molecular response of 415 de-novo CML patients in chronic phase enrolled in consecutive clinical trials of imatinib since July 2000 was examined. The assigned daily imatinib dose was 400 mg for 90 patients, 600 mg for 202 patients and 800 mg for 123 patients. Molecular data were included until imatinib cessation or last follow-up. The minimum time since commencing imatinib was 30 months and the median time on imatinib was 45 months, range 3 to 136. The CML8 PCR criteria for imatinib discontinuation were confirmed undetectable BCR-ABL1 transcripts at a sensitivity of 4.5 log that remained undetectable on all PCR tests for at least 24 months while on imatinib therapy. In the current analysis the CML8 PCR discontinuation criteria are defined as ‘stable UMR4.5'.
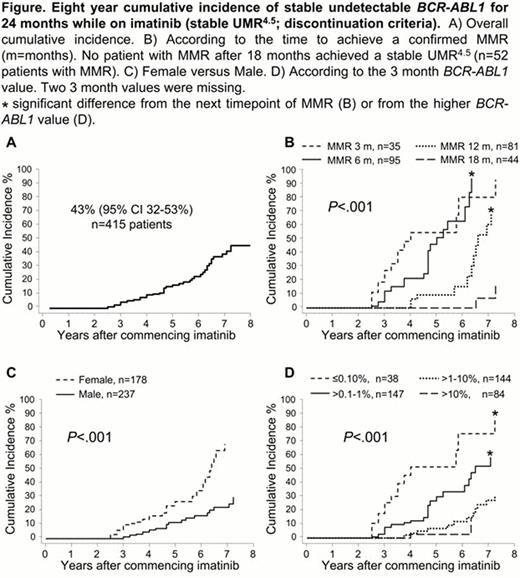
At 8 years of imatinib therapy the cumulative incidence of stable UMR4.5 was 43%, Figure A. Patients were divided into groups according to the time to a confirmed major molecular response (MMR): by 3, 6, 12 or 18 months. There was a significant difference in stable UMR4.5, P<.001, Figure B. The cumulative incidence of stable UMR4.5 was more than 60% for all patients who achieved MMR by 12 months and only 16% for patients with MMR between 12 and 18 months. The time to a confirmed MMR influenced the time to reach a stable UMR4.5 after achieving MMR. Considering only patients who achieved stable UMR4.5, patients achieving MMR by 3 months took a further 39 months (median) to achieve stable UMR4.5. For those with MMR by 6 months and 12 months, the median month to a stable UMR4.5 was 50 and 76 months after MMR, P<.001. This suggests slower dynamics of BCR-ABL1 decline with delayed time to MMR. 52 patients achieved MMR after 18 months and none achieved a stable UMR4.5 by 8 years: median time to MMR was 27 months, range 21–87. Factors at the time of commencing imatinib (baseline) were examined for their association with stable UMR4.5; Sokal risk, age, sex, assigned imatinib dose and baseline BCR-ABL1 value, as well as the 3 month BCR-ABL1 value. Quantitative factors were categorized into groups, with cut-offs set at the median for age and quartiles for the baseline BCR-ABL1 value. By univariate analysis the only baseline factor that predicted for higher cumulative incidence of stable UMR4.5 at 8 years was female versus male, 68% versus 30%, P<.001, Figure C. During imatinib therapy females had significantly lower median BCR-ABL1 values at every assessment up to 42 months. The 3 month BCR-ABL1 value also predicted stable UMR4.5, P<.001, Figure D. Baseline and 3 month factors were entered into a multivariate analysis. The 3 month BCR-ABL1 value and sex were independent predictors of stable UMR4.5, P=.004 and P=.005, respectively.
The time to achieve an MMR, sex and the 3 month BCR-ABL1 value predicted stable undetectable BCR-ABL1 while on imatinib. Lower BCR-ABL1 values and higher rates of stable UMR4.5 in females could be related to better drug adherence or biological differences. Further studies are indicated. Early MMR led to early achievement of the discontinuation criteria. The findings justify the focus on early achievement of MMR as a strategy to maximize recruitment to discontinuation studies.
Branford:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding; Ariad: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cepheid: Consultancy. Ross:Novartis: Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria. Yeung:Novartis Pharmaceuticals: Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding. Hughes:Novartis, Bristol Myers-Squibb, and ARIAD: Honoraria, Research Funding.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal