Abstract
Abstract 1646
Darinaparsin is a novel organic arsenical compound with potent anti-neoplastic activity. Preliminary clinical studies reported encouraging activity in patients with relapsed/refractory TCL and HL. We investigated the molecular mechanisms of action of darinaparsin in HL and TCL cell lines, the degree and method of cell death, and we investigated rational drug combinations for enhancement of therapeutic activity.
TCL (Jurkat, Hut78, HH) and HL cell lines (L428, L540, L1236) were treated with darinaparsin at increasing time and concentrations. MAPK, AKT/PI3K, mTOR and relevant pathways were analyzed by Western blot. Cell viability was assessed by MTT and apoptosis with Annexin V/PI flow cytometric analysis; this was confirmed by Western for caspase activation and PARP cleavage. In vivo tumor growth inhibition and survival of SCID mice was determined using xenografts derived from Jurkat (TCL) or L540 (HL) cell lines. The in vivo studies started with 7–8 mice for each control and treatment group. Once tumor volume average reached 100–250 mm3, treatment groups were injected (5 days/week) with darinaparsin SQ daily for 3 weeks. For drug combinations, varied novel agents were rationally combined with darinaparsin (BEZ-235 [PI3K/mTOR dual kinase inhibitor], PXD101 [HDAC inhibitor], olaparib [PARP inhibitor], MG132 [proteasome inhibitor], MK-2206 [pan-AKT inhibitor], SP600125 [JNK inhibitor], SB203580 [p38 MAPK inhibitor] and PD98059 (anti-MEK small molecule). We also interrogated pertinent signaling pathways with shRNA stable knock outs (KO) +/− darinaparsin.
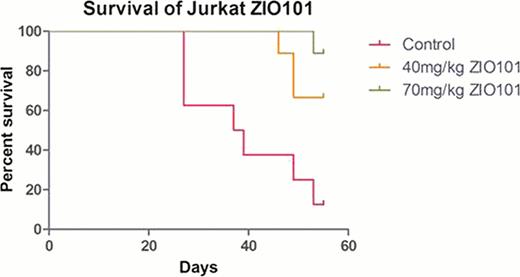
Treatment with 1–5μM Darinaparsin for 72 hours resulted in time- and dose-dependent cytotoxicity in all TCL and HL cell lines. IC50 observed with 72-hour treatment in the TCL lines Jurkat, HH, and Hut87 were 2.7μM, 3.2μM, and 6.7μM, respectively, and for the HL clines L540, L1236, and L428 were 1.3μM, 2.8μM, and 7.2 μM, respectively. Darinaparsin treatment also resulted in a dose dependent increase in apoptosis, detected by Annexin-V positivity and cleavage of PARP and caspases 3, 8 and 9 in all TCL and HL cell lines. In vivo experiments with lymphoma tumor xenografts derived from Jurkat showed significant inhibition of tumor growth (P<0.001) and improved survival (P<0.001) in darinaparsin-treated SCID mice compared with untreated control (see Figure); identical tumor reduction and survival data was noted in L540 xenografts. Next, we investigated relevant signaling pathways that were up- or down-regulated following darinaparsin exposure. We identified activation of the PI3K, MAPK, and mTOR pathways in all TCL and HD lines, expect for lack of MAPK response in L540; activation of pAKT was observed only in TCL cell lines (Jurkat and HH). Additionally, a concomitant decrease in levels of the inhibitory phosphatases (PTEN, SHP1) associated with AKT and MEK/ERK pathways were apparent in all TCL and HL cell lines treated with darinaparsin. Next, we tested cytotoxicity of darinaparsin (by MTT) in MEK and ERK KO using stably transfected shRNA in L540, Hut78 and Jurkat lines. There was minimal effect of these KOs in Jurkat, while there was increased cytotoxic effect with ERK shRNA in L540 and MEK shRNA in Hut78. Finally, we performed combination studies in L540 and Jurkat cell lines using the aforementioned novel targeted agents combined with darinaparsin in order to identify potential syngerism. Interestingly, the most notable combination was the PI3K/mTOR dual kinase inhibitor, BEZ-235, combined with darinaparsin; this resulted in synergistic cell death in Jurkat TCL cells (combination indices [CI]=0.5) and L428 HL cells (CI=0.5) cells as confirmed by MTT, Annexin-V and cleavage of PARP and caspases.
Collectively, these data show that the novel organic arsenical, darinparsin, induced significant cell death in TLC and HL cell lines and in lymphoma SCID xenograft models. Cell death with darinaparsin appeared to be dependent in part on the MAPK pathway, however this was cell line dependent. Additionally, we identified synergistic cell death in TCL and HL cell lines when darinaparsin was combined with a PI3K/mTOR dual inhibitor. Continued study of darinaparsin in TCL and HL is warranted either as a single agent or through co-targeting with PI3K/mTOR-based therapy.
Darinaparsin treatment of Jurkat (TCL) xenograft in SCID mice results in significant tumor reduction (volume) and improved survival.
Darinaparsin treatment of Jurkat (TCL) xenograft in SCID mice results in significant tumor reduction (volume) and improved survival.
Off Label Use: Darinaparsin for the treatment of T cell lymphoma and Hodgkin's disease.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal