Abstract
Abstract 1622
Contemporary therapy for mantle cell lymphoma (MCL) in fit patients usually consists of an induction phase followed by autologous stem cell transplantation (SCT). We previously reported a phase II trial of intensive chemotherapy induction with R-MACLO-IVAM followed by thalidomide maintenance and demonstrated promising progression-free survival (PFS) and overall survival (OS) rates without SCT (Lossos et al, Leuk Lymph 2010). We subsequently modified the protocol to utilize rituximab maintenance instead of thalidomide. Herein we present updated results and follow-up for the patients treated on this trial.
This was a prospective single-arm phase II trial conducted at the University of Miami with IRB approval. Eligible patients were chemotherapy-naïve and had a confirmed diagnosis of MCL using WHO criteria, age 18–75 years, ECOG PS 0–2, adequate organ function and no history of HIV or prior cancer. Pretreatment staging include CT and PET scans, endoscopy, colonoscopy and bone marrow biopsy. Cycle 1 consisted of R-MACLO (rituximab, methotrexate, doxorubicin, cyclophosphamide and vincristine) followed by G-CSF. When the ANC was >1.5×109/L, cycle 2 with R-IVAM (rituximab, ifosfamide, mesna, etoposide and cytarabine) was begun, followed by G-CSF, as previously reported. Fourteen days after ANC recovery from cycle 2, cycles 3 and 4 were given in identical fashion to 1 and 2. Four weeks after ANC recovery from cycle 4, patients were re-staged and responses were assessed by standard criteria. Patients who achieved complete responses (both radiologically and pathologically) were eligible for the maintenance phase. The first 22 patients were treated with thalidomide maintenance (target daily dose of 200mg); the protocol was subsequently modified to utilize rituximab maintenance at a dose of 375 mg/m2 IV weekly × 4 weeks, repeated every 6 months. Maintenance therapy was continued until MCL relapse or intolerable toxicity. OS was calculated from the date of diagnosis to the date of death or last follow up. PFS was calculated from date of diagnosis until the date of pathological evidence of recurrence or death. Data were summarized using descriptive statistics and survival was analyzed using the Kaplan-Meier method.
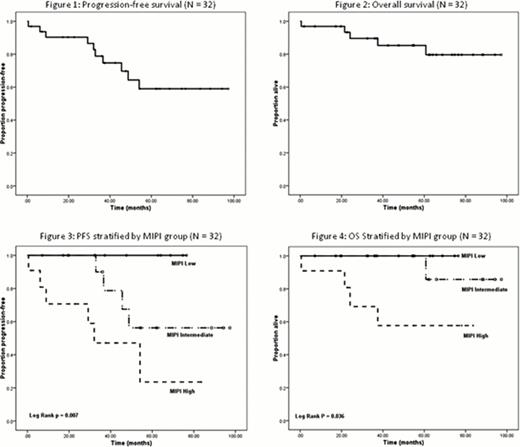
Between June 2004 and June 2012, 32 patients were enrolled, 22 on the first phase and 10 after the amendment to rituximab maintenance. All patients were evaluable for toxicity and 31 were evaluable for response. Median age was 56.5 years (range 39–73). All subjects had at least stage III disease with bone marrow involvement in 84% and gastrointestinal involvement in 38%. Distribution according to MIPI: low 28%; intermediate 38%; high 34%. All patients had diffuse variant except 2 with blastic variant. Twenty-eight patients completed all 4 cycles of therapy; treatment was stopped in 2 after 3 cycles, and in one after 2 cycles, and 1 died during cycle 1. Of the 31 patients completing 2 cycles of chemotherapy, 29 (94%) achieved a complete response (CR) and 2 had a partial response (PR). After a median follow-up of 54.9 months, the 5-year PFS was 69% (95% CI 51% – 82%) and the 5-year OS was 88% (95% CI 72% – 95%) [Fig 1 & 2]. Lower MIPI group (low vs intermediate vs high) was associated with longer PFS (log rank p = 0.007) and longer OS (log rank p = 0.036) [Fig 3 & 4]. Nine patients relapsed and 5 died; 1 died from sepsis on cycle 1; 1 died in CR at 38 months from non-small cell lung cancer diagnosed 19 months after MCL and the other 3 died from relapsed MCL after 22, 24 and 60 months respectively. Seven of the 9 patients who relapsed were treated with rituximab and bendamustine and one underwent an allogeneic SCT. Toxicities during the chemotherapy phase for the last 10 patients were similar to what was previously published for the first 22 patients, with the exception of lower renal toxicity since the dose of methotrexate was reduced to a total of 4.2 g/m2 instead of 6.7 g/m2 prior to the amendment. The toxicities during the thalidomide maintenance phase were similar to what was previously reported. For the 10 patients who received rituximab maintenance, there were no unexpected toxicities during the maintenance phase.
R-MACLO-IVAM results in a high overall response rate (94% CR and 6% PR) and a low relapse rate after over 4.5 years of median follow-up. The median PFS and OS still have not been reached. These results compare favorably with regimens that include upfront SCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal