Abstract
Abstract 1604
EBV (+) DLBCL of the elderly is a lymphoproliferative disorder recently included as a provisional entity in the 2008 WHO classification of lymphoid tumors. Defined as EBV (+) DLBCL in patients over the age of 50 years without known immunosuppression, information is evolving in this entity. To date, the true incidence of EBV (+) DLBCL in adults remains imprecisely determined and may vary regionally. This study sought to determine the incidence, presenting clinical characteristics, and outcome of EBV (+) in an unselected cohort of DLBCL patients enrolled in a prospective observational study.
The University of Iowa/Mayo Clinic SPORE Molecular Epidemiology Resource (MER) is a prospective, longitudinal observational study designed to collect information on patterns of care and outcomes for patients with newly diagnosed lymphoma. Patients seen at the Mayo Clinic, Rochester MN and the University of Iowa within 9 months of their initial diagnosis of lymphoma are offered enrollment. HIV (+) patients are excluded. Baseline patient reported data, clinical data and initial management are collected using a standard protocol and a central pathology review is performed. After enrollment, patients are actively followed for events (disease progression, retreatment, and death) and disease related comorbidities. Tissue Micro Arrays (TMA) are constructed on available primary biopsy specimens. Epstein Barr Encoded RNA (EBER) EBV testing was performed by in-situ hybridization, and scored by an expert hematopathologist. EBER reactivity in more than 30% of malignant cells were required for EBV(+) designation. All DLBCL patients from the MER with available TMA excluding primary mediastinal DLBCL and post transplant lymphoproliferative disorders (PTLD) are included in the current analysis. Event free survival (EFS) was defined as time from diagnosis until recurrence, retreatment, or death due to any cause.
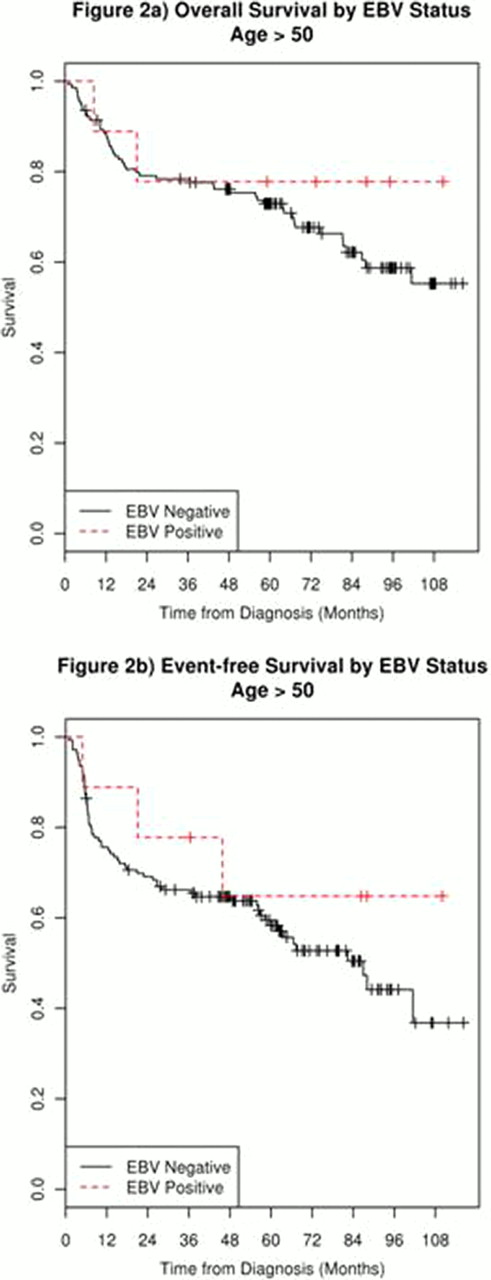
184 newly diagnosed DLBCL patients with available TMAs were enrolled in the MER from 2002–2009. The median age at enrollment was 62 (range 18–89) and 57% were male. 89.7% were treated with immunochemotherapy. At a median follow-up of 66 months (range 1–116), 80 patients (43%) had an event which included 59(32%) deaths. TMA testing was positive for EBV in 13 (7 %) samples with an even distribution among age groups. There was a trend toward higher percentage of stage III/IV disease (85 vs 57%, p = 0.052), and BM involvement (46 vs 16%). EBV (+) was not associated with other extranodal disease or further identifiable differences in presenting clinical characteristics. Controlling for IPI, the hazard ratio for EFS among EBV+ in DLBCL pts over 50 was 0.41 (95% CI = 0.13–1.32) p = 0.14. EFS and OS curves for pts over 50 are shown below. There were no events or deaths in the 4 EBV+ pts under age 50.
EBV+ DLBCL represents a small proportion of DLBCL patients presenting for care to our academic medical centers, consistent with reports from other regions. Substantial unique clinical characteristics at presentation for those pts with EBV+ disease were not identified, and outcomes were no worse than EBV (−) counterparts. Given the apparent regional differences in reports of EBV(+) DLBCL of the elderly, future clinical series will need to establish a more precise definition or determine more distinctive features to justify adoption of EBV(+) DLBCL of the elderly as a full entity in the WHO classification.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal