Abstract
Abstract 1526
The risk of developing Epstein-Barr Virus (EBV)-associated classical Hodgkin Lymphoma (cHL) shows an association with class I human leukocyte antigen (HLA) genes. HLA-A*01:01 is associated with increased risk of EBV-associated cHL whereas HLA-A*02:01 is associated with decreased risk. (Hjalgrim et. al, PNAS, 2010).The effects of these two alleles are independent of each other and the effect associated with each allele is additive i.e., possession of two HLA-A*01:01 alleles is associated with greater risk than one allele. HLA-A genes are involved in cytotoxic T cell (CTL) responses to exogenous antigens, and these data suggest that EBV-specific CTL responses are important in the pathogenesis of EBV-associated cHL. This study aimed to determine if HLA-A*01:01 and A*02:01 alleles are a factor in determining clinical outcome in EBV-associated cHL.
Patients with cHL were selected from two previous studies: the Scotland and Newcastle Epidemiological Study of Hodgkin's disease (SNEHD) (Jarrett et. al, Blood, 2005) and the “Investigation of the pathogenesis of Hodgkin lymphoma” (IPHL), an ongoing collection of patients principally from the West of Scotland. Both studies have ethical approval. Clinical follow-up data were obtained through clinical records, liaison with treating physicians or the Scotland and Newcastle Lymphoma Group (SNLG) database. Clinical treatment decisions were made by individual physicians. Cases were selected on the basis of availability of tumour EBV status, a suitable sample for HLA typing and clinical follow-up data and included 257 cases from the SNEHD study and 128 from the IPHL. HLA genotyping was performed to an intermediate resolution using allele-specific PCR (Gen-probe) and subsequent bead-based sequence specific oligonucleotide (SSO) assay (Luminex). Allele assignment was performed using Quicktype for Lifematch software (Gen-probe). Results were analysed for all cases, EBV-associated cases only, and EBV-negative cases only. Overall survival (OS) and Event-free survival (EFS) were analysed by number of HLA-A*01:01 alleles, number of HLA-A*02:01 alleles, and by the presence or absence of HLA-A*01:01 or HLA-A*02:01. Statistical analyses used Kaplan-Meier survival and Cox-regression analyses, adjusting for histological subtype, clinical stage, age and sex, and were implemented using SPSS v.19.
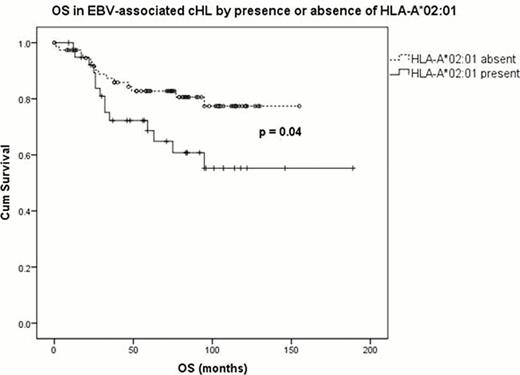
385 cases diagnosed between 1993 and 2008 were included in the analysis. 55.8% of cases were male and 128 (33.2%) had EBV-associated disease. Median follow up was 74 months (range 0–220 months). Clinical stage was available for 289 patients: stage 1 (17.9%); stage 2 (41.8%); stage 3 (22.5%); and stage 4 (17.6%). In the analysis of all cases there were no significant differences in OS and EFS by number or presence of HLA-A*02:01 and A*01:01 alleles. EBV-associated cHL cases demonstrated inferior OS with the presence of HLA-A*02:01 (p=0.04) (figure). This was corroborated by the demonstration of inferior OS with increasing number of HLA -A*02:01 alleles present (p=0.016). Increasing number of HLA -A*02:01 alleles was also associated with inferior EFS but differences were not statistically significant. HLA-A*01:01 was not associated with OS or EFS in EBV-associated cHL. There was no significant association between histological subtype and OS or EFS. When clinical stage, age, and sex were included in the Cox-regression analysis of survival in EBV-associated cases, only age (p<0.001) was significant; presence of an HLA-A*02:01 allele approached statistical significance (p =0.062).
In this study the HLA-A*02:01 allele was associated with inferior survival in EBV-associated cHL. As HLA-A*02:01 is associated with a decreased risk of developing EBV-associated cHL, we hypothesise that cHL arising in this 'protected' group is associated with greater biological or immunological dysfunction, resulting in poorer outcome. HLA-A genotype may be helpful in identifying patients who have inferior outcomes using standard therapy, and who may benefit from novel or cellular therapies. Larger studies within the context of clinical trials are required to extend these findings. In addition, this study supports the importance of stratifying cHL cases by EBV-status in clinical studies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal