Abstract
Abstract 1472
Despite significant advances in survival rates for pediatric Acute Lymphoblastic Leukemia (ALL) patients (pts), long-term survival rates for adults with ALL have remained below 40%. An absolute lymphocyte count (ALC) < 350/μL at Day 28 of induction therapy is predictive of poor outcome [event-free survival (EFS) and overall survival (OS)] in adults with newly diagnosed ALL. It is unknown, however, whether ALC at Day 28 is also predictive of outcome in those patients who undergo allogeneic HCT in CR1. We hypothesized that the prognostic impact of ALC at Day 28 might be nullified by HCT in CR1 due to the graft versus leukemia effect.
We conducted a retrospective chart review of 90 adult pts (≥ 18 yrs of age) with de novo ALL who underwent HCT while in CR1 during the years 1998–2011 at the Cleveland Clinic and Stanford, 66 of whom were evaluable for data analysis. Institutional review board approval was obtained at each institution. Prior studies identified an ALC of 350/ μL at Day 28 of induction therapy as a cut-off predictive of outcome. Therefore, we evaluated this number as well as other cut-offs. Cytogenetic (CG) risk was ascribed by CALGB criteria. We also evaluated the impact of gender, age at diagnosis, CG, and WBC at diagnosis. The Kaplan-Meier method was used to summarize OS and EFS, which were measured from HCT to death and the first of relapse/death, respectively. The log-rank test was used for univariable analysis of categorical factors and the Cox proportional hazards model, stratified by institution, was used for multivariable analysis and univariable analysis of measured factors.
Median age: 38 yrs (range 19–66); Gender: 58% (38) male; CG risk: 57 (86%) poor, 5 (8%) miscellaneous, 4 (6%) normal; 64 (97%) B-cell lineage; median WBC at diagnosis 18.1 K/μL (range 0.9–432); median Day 28 ALC 440/μL (range 0–2450). The majority of pts (50, 76%) received a vincristine/prednisone/anthracycline-based induction regimen, with the remainder receiving a high-dose cytarabine based regimen. The median interval between the start of induction and CR was 28 days and the median interval from CR to HCT was 3.2 months (0.2–10.8).
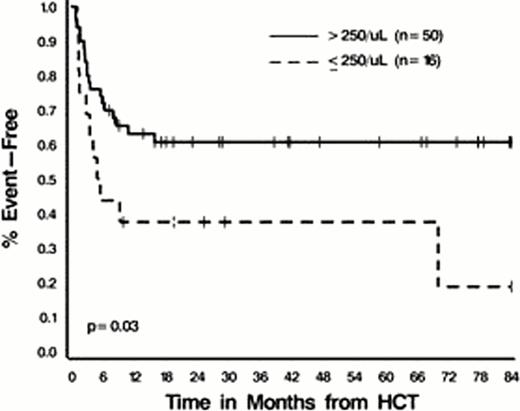
Median EFS and OS have not been reached; 1-year EFS is estimated to be 57% ± 6%, and 2-year OS 57% ± 7%. In univariable analyses age > 50 (EFS) and WBC at diagnosis ≥ 40 K/μL (EFS and OS) were associated with worse outcomes (all p ≤ 0.04). A Day 28 ALC < 250/μL (but not < 350/μL) was also associated with decreased EFS [HR 2.27 (1.08–4.79), p=0.03] (Figure 1) with a trend towards worse OS [HR 1.89 (0.89–4.04), p=0.10]. WBC and Day 28 ALC remained statistically significant prognostic factors for RFS on multivariable analysis; HRs 2.89 (1.38–6.05), p=0.005 and 2.25 (1.06–4.78), p=0.04, respectively.
ALC at Day 28 remains prognostic for outcome in newly diagnosed ALL pts undergoing HCT in CR1. Further characterization of the lymphocytes in pts with high ALCs and their potential role in preventing relapse is needed.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal