Abstract
Abstract 1436
The distinction in antigen expression between normal T cells and T-ALL is currently limited by a narrow breadth of clinically useful antibodies. Our study aimed to enhance the diagnostic utility of flow cytometry in T-ALL diagnosis and minimal residual disease (MRD) detection by identifying novel aberrant antigen expression in T-ALL.
A high throughput flow cytometric screening method containing 242 antibodies (Lyoplate, Becton Dickinson) was used to evaluate 3 normal peripheral blood specimens and 9 diagnostic paediatric T-ALL cases. An LSRII (Becton Dickinson) was used for all flow acquisition, and Woodlist software for all analysis. The initial screening panel comprised a 9 colour assay (1 detection and 8 gating fluorochromes). All Lyoplate antibodies were analysed for differences in median fluorescence intensity between normal T cells and T-ALL populations. Subsequently, 9 specific novel antibodies were chosen for expanded testing in prospectively enrolled paediatric T-ALL cases on Children's Oncology Group protocols, using 3 additional tubes. A 9 color flow cytometric assay was designed with the following backbone antibody combination: CD3 PE-TR, CD16+CD56 PE-Cy5, CD5 PE-Cy7, CD7 V450, CD45 KO. Additionally, in each tube one of the following combinations was utilized: CD226 FITC, CD184 PE, CD229 APC or CD6 FITC, CD53 PE, CD200 APC or CD244 FITC, CD165 PE, CD27 APC. Median fluorescence intensites were compared between T-ALL populations and normal donor T cells, and the ratio of abnormal T-ALL: normal donor T cells calculated for each antibody.
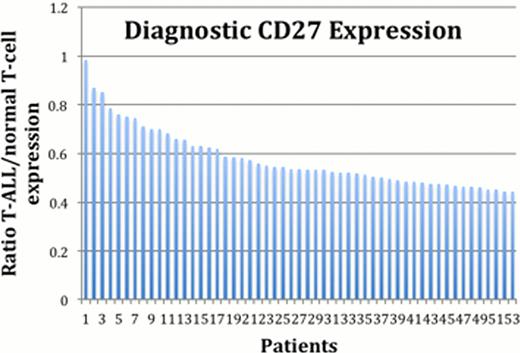
A total of 59 patients with T-ALL were analyzed median age 8 (2–29) with 38 males, including 6 early thymic precursor (ETP) cases by St Jude's criteria. In total, 94 episodes were included: 59 pretreatment, and 35 post treatment cases at Day 29. In the T-ALL, CD27 expression was significantly reduced with a median blast/normal T cell ratio of 0.54 at diagnosis, and 0.52 post treatment. 48/53 (91%) patients had a reduced ratio <0.75 at diagnosis and 18/31 (58%) post treatment. Additionally CD6, CD226 and CD200 showed a mild reduction at diagnosis of 0.75, 0.81 and 0.84 respectively, with similar ratios seen with these 3 antibodies post treatment. The ETP cases showed a significant reduction in CD27 expression, median ratio 0.44, while reduced expression was also seen with CD6 (0.72), CD165 & CD184 (0.77), CD53 (0.78) and CD226 (0.80). There was no difference in the ratios calculated when using internal patient T cells versus normal donor T cell expression levels.
We have demonstrated that expression levels of CD27 are consistently reduced in the majority of T-ALL and ETP patients at diagnosis and expression levels are stable at post treatment assessment. This marker has potential utility in clinical practice for diagnosis and MRD assessment. A spectrum of additional antibodies also demonstrated reduced expression, especially in ETP cases, however further patient recruitment and evaluation is required for more definitive evaluation of the utility of these antigens.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal