Abstract
Abstract 1415
Chronic myeloid leukemia (CML) is one of the best examples of a disease that can be targeted by molecular therapy however, the success of new designed drugs is largely restricted to the chronic phase of the disease. If not cured at this stage, CML invariably progresses and transforms into an acute-type leukemia undegoing a blast crisis, that is characterized by a rapid expansion of myeloid or lymphoid differentiation-arrested blast cells leading to short median survival. To investigate the genetic changes associated with CML progression under tyrosin-kinase inhibitor treatment, and to determine whether clonal evolution contributes to blast crisis, we performed whole-exome sequencing of an individual patient at three different times of the CML progression: chronic phase (CP), complete hematological remission (CHR) and blast crisis (BC).
We collected genomic DNA from bone marrow cells (tumor DNA) at three disease evolution points and from epithelial cells (germline DNA). The sequence capture, enrichment and elution was performed according to manufacturer's instructions and protocols (SureSelect, Agilent). Each eluted-enriched DNA sample was sequenced on an Illumina GAIIX as paired-end 75b reads. The bioinformatics analysis of sequencing data was based on a pipeline which includes alignment, annotation, and filtering of the somatic variants, all linked to a coverage/depth statistical analysis. Validation of somatic mutations was done by capilary sequencing.
The patient, a 65 year-old men, showed at diagnosis a classic CML with a 45,XY,t(9;22)(q34;q11.2),rob(13;14)(q10;q10)c [20]. He was treated with Imatinib (400 mg/day) achieving complete hematological and cytogenetic remission after 12 months of treatment. Real-time qRT-PCR demonstrated molecular response: BCR/ABL1 ratio decreased from 53% to 13% within the first year. Unfortunately, the disease progressed at month 14 to a blast crisis with a complex karyotype that did not respond to 2nd line treatment (Dasatinib + Idaurobinice-AraC) and the patient died of the disease 18 months after diagnosis.
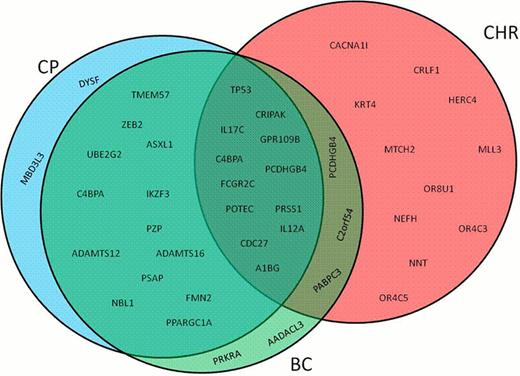
After discarding the variants present in the matched normal DNA and in the dbSNP132 database, we obteined a total of 3123, 7678 and 3306 single nucleotide substitutions (SNSs) and small insertions and deletions (indels) for CP, CHR and BC, respectively. Next, we selected only those variants within coding regions that, passing depth and quality controls, were predicted to produce non-synonymous amino acid changes. This resulted in 27, 30 and 26 SNSs for CP, CHR and BC, respectively, (Fig. 1).
Among those SNSs, we validated mutations in genes known to be involved in CML (such as ASXL1 and TP53) as well as in genes that have not been described so far in the disease (such as UBE2G2, ZEB2 and IKZF3). TP53 mutation (p.E286K) was found in the three phases of CML progression. However, ASXL1 (p.G679*), UBE2G2 (p.D35V), ZEB2 (p.L420R) and IKZF3 (p.E272K) were present only in the CP and BC-CML. On the other hand, only 7%, 42% and 6% of the mutated gene were exclusively found in CP, CHR and BC, respectively (Fig. 1).
The evaluation of the number of mutated reads for each gene allowed us to study clonality and clonal evolution patters during CML progression. 93% of the selected SNSs that were present in the CP were also seen in BC (only 46% during CHR). In fact, the percentages of reads of the mutant alleles identified for the most relevant genes were the same (around 50%) both at CP and at BC.
Whole-exome sequencing allowed to identify a large number of mutated genes, even at the chonic phase of CML, that harbour clear prognostic and predictive significance (TP53, IKZF3, absence of ABL1 mutations). The study of the mutation profile through the disease progression indicated that, at least in this patient, the number and the type of mutations were rather similar at CP and BC.
Mutated genes distribution in chronic myeloid leukemia progression. CP- chronic phase; CHR- complete hematological remission; BC- blast crisis.
Mutated genes distribution in chronic myeloid leukemia progression. CP- chronic phase; CHR- complete hematological remission; BC- blast crisis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal