Abstract
Abstract 1360
The tyrosine kinase inhibitor (TKI) imatinib is used as the first-line therapy for newly diagnosed chronic myeloid leukemia (CML). However, some patients fail to respond or become intolerant to imatinib. Nilotinib is a second-generation TKI with higher selectivity and more potent inhibitory effects on BCR-ABL than imatinib. Several studies have shown hematologic and cytogenetic responses to nilotinib in patients with imatinib-resistant or intolerant CML.
To investigate the safety and efficacy of nilotinib for patients with imatinib-resistant or intolerant, chronic (CP)- or accelerated (AP)-phase CML from the East Japan CML Study Group (EJCML) trial by evaluating molecular responses in terms of the BCR-ABL1 mutational status and plasma trough concentration of nilotinib.
In this multicenter phase II clinical trial, nilotinib (400 mg bid) was administered orally for one year and the molecular responses were monitored by means of the international scale of quantitative PCR (IS-PCR). BCR-ABL1 mutations were analyzed by direct sequencing at the baseline and 12 months or at the time of the event for discontinuation of the treatment (i.e., progressive disease, insufficient effects, or severe adverse events). The plasma trough concentration of nilotinib was measured by high-performance liquid chromatography 3 months after nilotinib administration.
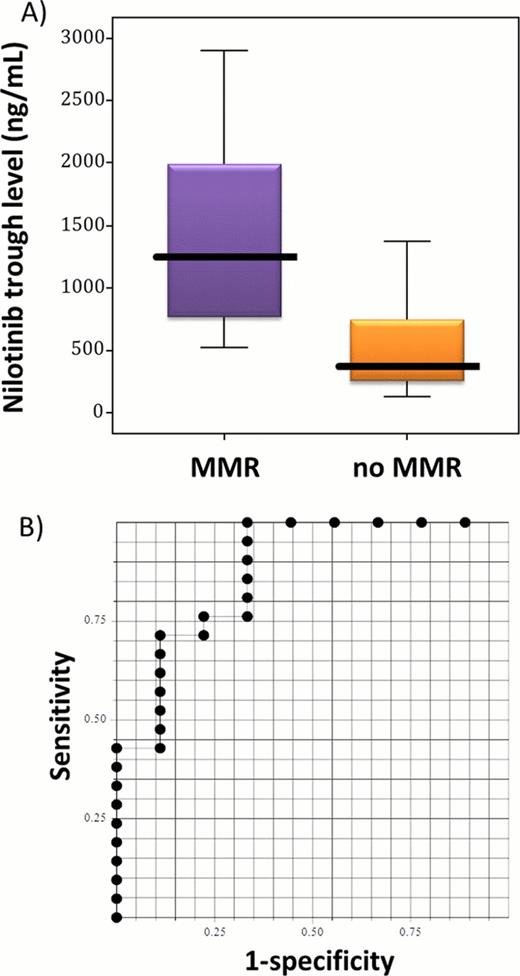
From March 2009 through February 2011, 51 patients were registered in this study, and data of 49 patients whose molecular responses were evaluated by the IS-PCR were analyzed (imatinib-resistant CML = 33, imatinib-intolerant CML = 16; CP CML = 46, AP CML = 3). The median follow-up period was 12.0 months (range = 0.1–13.3 months). At 6 and 12 months, the major molecular response (MMR; ≤0.1% IS) rates were 52.5% and 67.6%, respectively, and the complete cytogenetic response (CCyR)-equivalent (≤1.0% IS) rates were 75.0% and 85.3%, respectively. Five types of BCR-ABL1 mutations (M244V, F317L, N358D, F359V, and E459K) were detected in 6 patients (12.2%) at the baseline, but the M244V, N358D, and E459K mutations disappeared after the nilotinib treatment. Acquired BCR-ABL1 mutations (Y253H, I418V, and exon 8/9 35bp insertion) were detected in 3 patients (8.6%) at 12 months or at the time of the event; these patients did not achieve a CCyR or an MMR. No patients showed an acquired mutation of T315I. Most patients except 11 subjects (22.4%) still received the treatment. The reasons for discontinuation were progressive disease in one patient with an F317L mutation, insufficient effects in one patient without any mutation, and adverse events in 9 patients (thrombocytopenia in 5 patients, hyperbilirubinemia in 2 patients, headache in one patient, and heart disease in one patient). Among 30 patients without BCR-ABL1 mutations, the plasma trough concentration of nilotinib was significantly higher in 21 patients with an MMR than in those without an MMR by 12 months (median = 1255.1 ng/mL vs. 372.8 ng/mL, P = 0.0012 by Mann–Whitney U-test; see the figure). The concentration of 761 ng/mL was significantly associated with an MMR by 12 months in a receiver-operating characteristic (ROC) curve analysis of the best sensitivity (76.2%) and specificity (77.8%).
The patients with imatinib-resistant or intolerant, CP or AP CML, even those having BCR-ABL1 mutations M244V, N358D, and E459K, achieved an MMR by 12 months of nilotinib treatment. The plasma trough concentration of the drug was related to the MMR by 12 months, and the plasma threshold of nilotinib should be set above 761 ng/mL. These findings suggest that nilotinib shows good efficacy and tolerability in Japanese patients with imatinib-resistant or intolerant, CP or AP CML. (ClicalTrials.gov, UMIN ID 000002201)
A) Relationship between the plasma trough concentration of nilotinib at 3 months and the major molecular response (MMR; ≤0.1% IS) by 12 months.
B) Receiver-operating characteristic (ROC) curve of the plasma trough levels of nilotinib and their potential for discriminating an MMR. The area under the curve is 0.87 ± 0.07.
A) Relationship between the plasma trough concentration of nilotinib at 3 months and the major molecular response (MMR; ≤0.1% IS) by 12 months.
B) Receiver-operating characteristic (ROC) curve of the plasma trough levels of nilotinib and their potential for discriminating an MMR. The area under the curve is 0.87 ± 0.07.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal