Abstract
Abstract 1177
Allogeneic blood transfusion is a potential source of infection via a variety of known and unknown transmissible agents. Over the last three decades, pre-transfusion donor screening for viral agents has led to a dramatic reduction in the risk of virally transmitted diseases. Bacterial contamination, on the other hand, has proved more difficult to address and remains the most prevalent transfusion-associated infectious risk. This is especially true for platelet components whose storage conditions (22°C, for up to 5 days, with agitation) facilitate bacterial proliferation throughout the storage period. Reported here are the results of initial testing using a novel, noninvasive, real time, rapid screening device for the detection of bacterial contamination of platelet units.
This detection method is based on measuring absorption of an infrared beam that is transmitted through the gaseous atmosphere above the platelets. Living microorganisms produce metabolic gases such as carbon dioxide (CO2) during respiration. By means of infrared absorption the concentration of metabolic gases can be measured inside the platelet storage bag.
The methodology consists of an apparatus which uses a tunable monochromatic mid-IR light source, IR detector and electronic signal processor. The light source emits light in frequency range overlapping at least with one absorption line of CO2gas. Use of the tunable light source allows the determination of metabolic gas concentration within the container without etalon use. In this method, the light from the light source is transmitted through the gaseous part of storage bag is measured by means of an IR detector. The concentration of CO2gas inside the platelet bag is determined by equilibrium conditions between the release rate and the rate of diffusion of the metabolic gases through the bag walls.
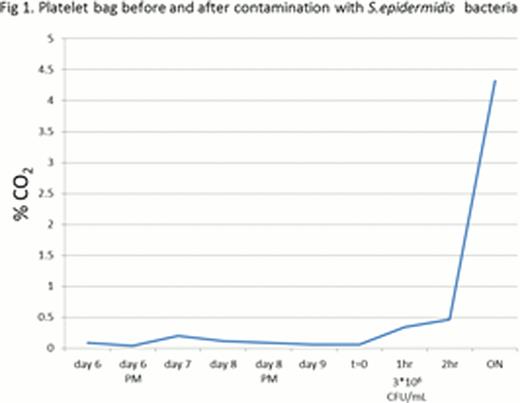
Staphylococcus epidermidis obtained from the American Type Culture Collection (ATCC) were used to contaminate platelets bags. The bacterially inoculated apheresis platelets were agitated at 22°C and measurements were performed using a laser instrument. Each platelet unit was measured before and during bacterial contamination. Samples were taken from each contaminated platelet bag and a standard culture plate count was used for determining bacterial concentration in the platelet medium. Using this device we have succeeded to detect bacterial concentration of above 3*106 CFU/mL staphylococcus epidermidis (Figure 1).
Although methods to detect platelet bacterial contamination have received much attention, bacterial contamination of platelet components remains a persistent problem. The methodology described in this report detects staphylococcus epidermidis in apheresis platelet bags. The method allows for testing in real time - at issue or during storage, and it provides immediate results.
This device is expected to be successful in detecting most prevalent types of bacteria strains. The test is easy to perform and does not require pre-incubation of samples or handling of the bag's contents. The device is specific and sensitive, allowing bacteria screening, ensuring increased safety of platelet transfusions. The device is able to detect bacteria in platelets, and other blood constituents, through the storage bag, without contacting, harming, or handling the bag's contents. Since there is no direct interaction of the laser light beam with the platelet media and the laser power is low (approximately 10 mW) thermal effects are avoided.
By allowing real-time, sensitive detection of bacterial contamination of platelet products, wastage can be reduced, platelet shortage can be alleviated and the adverse outcomes associated with platelet transfusion contamination can be prevented. Further studies are required to evaluate the sensitivity limits for the detection of other bacterial strains that have been reported to contaminate platelet products.
CO2 level of apheresis platelets. Measurements of CO2 that accumulates above bacterially contaminated apheresis platelets were performed using a laser instrument. Expired apheresis platelets (6 days old) were examined before and after contamination with Staphylococcus epidermidis (t=0). CO2 concentration rose rapidly beginning one hour after inoculation (at a bacterial concentration of 3*106 CFU/mL).
CO2 level of apheresis platelets. Measurements of CO2 that accumulates above bacterially contaminated apheresis platelets were performed using a laser instrument. Expired apheresis platelets (6 days old) were examined before and after contamination with Staphylococcus epidermidis (t=0). CO2 concentration rose rapidly beginning one hour after inoculation (at a bacterial concentration of 3*106 CFU/mL).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal