Abstract
Abstract 1170
Many complications, particularly hematological ones, affect the outcome of children on extracorporeal membrane oxygenation (ECMO). In an effort to minimize clotting and bleeding complications, some pediatric ECMO centers administer antithrombin (AT) concentrate to augment heparin's anticoagulant activity. However, the impact on clinical outcomes is unclear.
To investigate whether intermittent AT concentrate administration improves outcome in pediatric ECMO patients
This is a retrospective cohort analysis of 64 youths aged 0 to 21 years who received ECMO for respiratory failure at a single institution between 1/2007 and 9/2011. Subjects either received 1+ dose of plasma-derived AT concentrate (Thrombate III®, Grifols) at the discretion of the attending critical care physician (“AT+” cohort), typically for an AT antigen level <80% with either a high unfractionated heparin (UFH) need or ex vivothrombosis, or did not (“No AT” cohort). Dose was calculated to obtain an AT antigen target level of 120% (IU = [(120-AT level) × weight (kg)]/1.4). AT antigen levels were assayed by an immuno-turbidimetric method (Liatest® ATIII, Stago).
Short-term increase in AT antigen levels was the primary endpoint. Secondary endpoints included UFH requirement, number of bleeding and thrombotic complications, number of ECMO circuit changes, length of stay and in-hospital mortality.
Of 64 subjects, 30 subjects in the AT+ cohort received a total of 77 AT doses (range 1–8 doses/pt) while the No AT cohort contained 34 subjects. For the AT+ and No AT cohort, respectively: median age was 0.1 (range 0–188) mos. vs 1.7 (0–250) mos. (p=0.21); median first AT level was 50.5% (15–75) vs 54% (19–108, p=0.48); and median ECMO duration was 180 (71–613) hrs vs 146 (44–1,467) hrs (p=0.76).
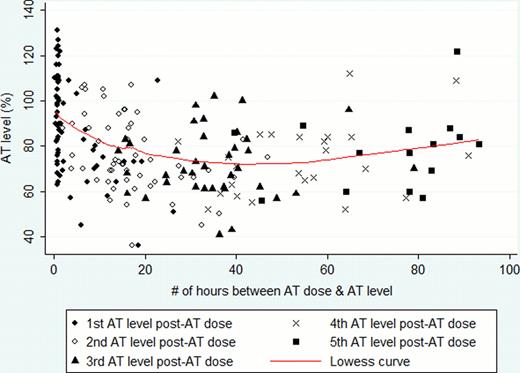
When comparing all AT levels drawn within 12 hrs of a prior measurement, AT antigen was on average 66% in the AT+ cohort compared to 42.2% in the No AT cohort [23.8% higher in AT+; 95% confidence interval (CI), 10.2 – 37.5%], adjusted for age, ECMO duration and first AT level. When comparing only the first AT level drawn within 12 hrs of a prior measurement after an AT dose, AT levels were on average 80.1% in the AT+ cohort compared to 41.7% in patients in the No AT cohort (38.4% higher in AT+; 95% CI, 36.1 – 45.2%). Only 6 of 77 doses reached the targeted AT level of 120% (8%). Of the 28 doses after which the AT level was followed sufficiently, median time to fall to an AT level of 80% was 6.8hrs after receiving the dose (mean: 9.8 hrs, Figure 1).
For the AT+ cohort, mean UFH rate decreased by 10.1 u/kg/hr for the 3hrs following the AT dose (95% CI, 7.6–36.6; p<0.001) compared to the 3hrs prior. The UFH rate remained significantly lower 12hrs following administration (10.2 u/kg/hr lower; 95% CI, 6.2–14.1; p=<0.001).
No significant differences existed in the number of patients with an in vivo thrombosis (14.7% vs. 13.3%, p=1.0); hemorrhagic complications (0.14 more bleeds in AT+ vs. No AT cohort; 95% CI, −0.34–0.63; p=0.56); number of circuit changes (0.15 changes more in AT+; 95% CI −0.002–0.001, p=0.88); median length of stay in the hospital (36.8d for AT+ vs. 49.8d for No AT, p=0.91) or intensive care unit (25.3d for AT+ vs. 34.1d for No AT, p=0.63), or adjusted relative risk for in-hospital mortality (0.6; 95% CI, 0.2–1.5).
Intermittent, on-demand dosing of AT concentrate in pediatric patients on ECMO for respiratory failure increased AT levels, but not typically to the targeted level. Following doses with a measurable AT level, the majority of AT levels fell to <80% by 6.8 hrs. The UFH rate remained lower than before the AT dose for > 12 hours. However, in this retrospective study, no differences were noted in the measured clinical endpoints. A prospective randomized study of this intervention may require different dosing strategies; such a study is warranted given the variable use of this costly product across institutions.
Off Label Use: Antithrombin concentrate. Gernsheimer:Amgen: Consultancy, Honoraria; Symphogen: Consultancy; Laboratorios Raffo SA: Honoraria; Clinical Options: Consultancy; Hemedicus Corporation: Honoraria; Glaxo Smith Kline Corporation: Consultancy; Shionogi Corporation: Research Funding; Cangene Corporation: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal