Abstract
Abstract 1165
Bivalirudin (BIV) is a direct thrombin inhibitor with a favorable pharmacologic profile in comparison to heparin. In a previous study in children <6 months of age with venous thromboembolism (VTE), bivalirudin was demonstrated to be a safe and potentially more effective anticoagulant. The present study's aims were to assess the safety, dosing, pharmacokinetics, pharmacodynamics, and efficacy of BIV in children >6 months of age.
This prospective, open-label study evaluated the use of BIV for initial treatment of a new VTE in children between 6 months and 18 years of age. Eligible subjects received a BIV bolus of 0.125mg/kg followed by an initial continuous infusion of 0.125mg/kg/hour which was adjusted as needed to achieve a PTT of 1.5–2.5x their baseline PTT. BIV levels by high performance liquid chromatography and PTTs were tested simultaneously 5 times in the first 24 hours, daily thereafter and following all dose adjustments. Subjects remained on BIV until they were transitioned to long-term anticoagulant treatments. Imaging was required to confirm the presence of a VTE and follow-up imaging was obtained at 2–3 days and 30 days following initiation of anticoagulation. Subjects were closely monitored for bleeding and adverse events.
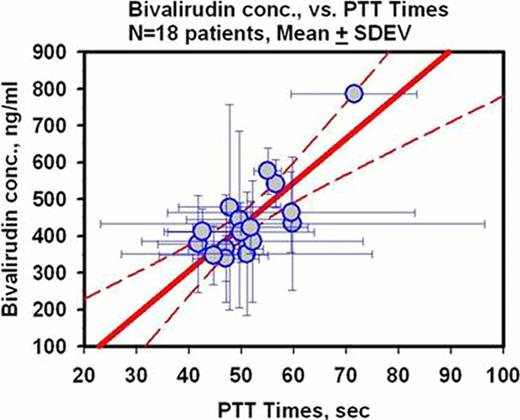
18 subjects (11M:7F) ranging in age from 9 months-17 years completed the study. There were no major bleeding events and one minor bleeding event. Outcome of VTE: at 2–3 days, 2 subjects had complete resolution and 7 subjects had partial resolution while at 30 days, 7 subjects had complete resolution and 9 subjects had partial resolution. There were no symptomatic recurrent events or any asymptomatic local progressions by surveillance imaging. Seven subjects required no dose adjustments during their course of treatment ranging between 2–5 days. See figure for correlation of PTT with BIV levels [each circle represents one subject with their mean PTT and BIV level with the standard deviations (r2=0.64)].
BIV offers a potentially safer, more effective and pharmacologically more predictable alternative to heparin for initial anticoagulation of children with VTE. Pharmacokinetic-pharmacodynamic correlation was excellent suggesting PTT as an acceptable monitoring strategy for BIV in this population. Further studies comparing BIV to heparin should be conducted.
Off Label Use: Bivalirudin is not approved for use in children. Goldenberg:Eisai Inc: Research Funding; Pfizer Inc: Membership on an entity's Board of Directors or advisory committees; CPC Clinical Research: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal