Abstract
Abstract 1090
Rituximab (RTX), a chimeric monoclonal immunoglobulin G1 anti-CD20, is an effective treatment of persistent or chronic immune thrombocytopenia (ITP) with a rate of initial response (6 to 12 months after infusion) of 40 to 60% (Godeau et al., Blood, 2008). However, this drug is not licensed in ITP and there is no clear predicting factor of response to RTX. The FCGR3A 158 V/F polymorphism is known to affect both FcgRIIIA expression, binding of RTX to FcgRIIIA, and RTX-mediated cytotoxicity (Hatjiharissi, Blood, 2007). Studies have reported that the FCGRIIIA V allele was associated to a better response to RTX in some B lymphoid malignancies (Cartron G et al, Blood, 2002), in rheumatoid arthritis (Ruyssen-Witrand A et al, Ann Rhem Dis, 2012), and also in hepatitis C related cryoglobulinemia (Gragnani L et al, Arthritis Rheumatism, 2011). The aim of the present study was to evaluate whether FCGR3A 158 V allele was associated with a better response-rate to RTX in adults with persistent ITP.
Every patient aged of 18 years and more, with a definite diagnosis of primary ITP (Rodeghiero et al.) treated by RTX, seen at one of the 3 participating centers over the study period (2008–2011) could be included. The response to RTX was assessed at 6 ± 2, 12 ± 2 and 24 ± 2 months after RTX first administration. A response (R) was defined as a platelet count between 30×109/L and 100 × 109/l with a least a twofold increase from baseline in the absence of any concurrent rescue or new medication, and a complete response (CR) was defined by a platelet count 3 100 × 109/L. FCGR3A V/F158 genotyping was performed by means of allele specific PCR SYBR Green as previously described (Dall'Ozzo et al., JIM, 2003). Response rates were compared across FCGR3A genotypes using the Fischer exact test and with a Log Rank analysis. A p value < 0,05 was considered significant.
A total of 103 ITP patient treated with RTX between 2008 and 2011 were included. Their main characteristics are listed in table 1. The mean follow-up after RTX was 43 ± 31 months, 90/103 patients were evaluable at 12 months and 68/103 at 24 months. The genotypic distribution of the FcgR3A V/F 158 polymorphism among ITP patients was not different from the one of 50 healthy controls of the same Caucasian origin, 35 (34%) patients were FF, 51 (50%) were FV, and 17 (16%) were homozygous VV. At 6 months after RTX, the overall initial response rate was 53% including 37 (36%) CR, and 18 (17%) R. At 12 months, among the 90 evaluable patients, 30 (61%) had a CR, 6 (12%) a R and 13 (27%) were non-responders (NR). At 24 months, a persistent CR was observed in 16/68 evaluable patients (24%) a R in 4 (6%) and 48 (71%) were considered as non-responders (primary failure, or relapse after initial response).
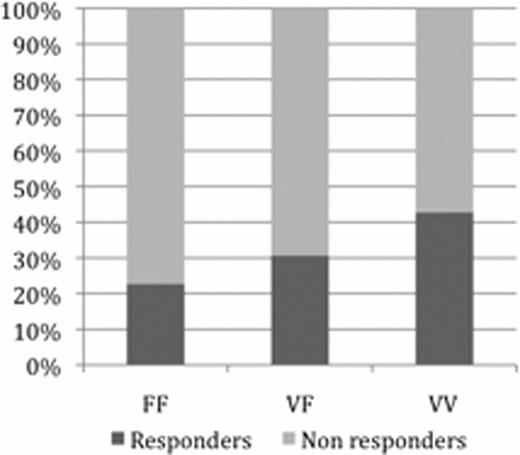
Although the response-rate was higher and longer in patients carrying the VV genotype, there was no significant difference in the genotypic distribution between responders and non-responders at 6, 12 and 24 months.
Although there was a trend towards a better response rate in patients with the VV genotype, this difference was not statistically significant. This result which is contradictory with other recently published data (Cooper et al., BJH, 2012), provides no evidence for recommending a FCGR3A genotype prior to RTX use in ITP to select the patients with a higher likelihood of response. ITP remains a heterogeneous disease which pathophysiology can be related on B and/or T cells abnormal autoreactivity. Identifying the subset of ITP patients with a B-cell dependant pathway could increase the interest of re-considering the impact of FCGRIIIA 158 V/F genotype for predicting the response of B-cell targeted therapies.
| Patients characteristics . | n (%) . |
|---|---|
| Age at RTX perfusion (mean) | 50 (18) |
| Sex, female | 73 (71) |
| Median disease duration, months | 56 (71) |
| Hemorrhagic score at diagnosis1 | 4.8 (4.3) |
| Severe hemorrhagic complication2 | 8 |
| Corticoid responders | 65 |
| Number of treatment lines before RTX3 (mean,number) | 1.2 (1.1) |
| Previous splenectomy | 13 |
| Positive antinuclear antibody | 12 |
| RTX infusion modality: 375 mg/m2 × 4 weeks/1 g every 2 weeks | 65/26 |
| Patients characteristics . | n (%) . |
|---|---|
| Age at RTX perfusion (mean) | 50 (18) |
| Sex, female | 73 (71) |
| Median disease duration, months | 56 (71) |
| Hemorrhagic score at diagnosis1 | 4.8 (4.3) |
| Severe hemorrhagic complication2 | 8 |
| Corticoid responders | 65 |
| Number of treatment lines before RTX3 (mean,number) | 1.2 (1.1) |
| Previous splenectomy | 13 |
| Positive antinuclear antibody | 12 |
| RTX infusion modality: 375 mg/m2 × 4 weeks/1 g every 2 weeks | 65/26 |
Khellaf et al., Haematologica, 2005.
including digestive hemorrhage or any severe external bleeding.
including dapsone, danazol, vincristine, vinblastine, hydroxychloroquine, aziathropine, mycophenolate mofetil, eltrombopag.
RTX response rate according to FCGR3A genotype at 6 (A), 12 (B) and 24 (C) months
RTX response rate according to FCGR3A genotype at 6 (A), 12 (B) and 24 (C) months
Viallard:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal