Abstract
Abstract 1064
Adenosine Di-phosphate (ADP) is stored in dense granules of platelets and is released upon platelet activation acting as a feedback activator by binding to G-protein coupled P2Y1 and P2Y12 receptors. ADP stimulation causes platelets to change shape, aggregate, release dense and a-granule contents and synthesize thromboxane A2 that can further act as a feedback activator potentiating platelet responses by binding to thromboxane receptor (TP). Protein kinase C is a serine threonine specific kinase that regulates multiple platelet functional responses. Specific PKC isoforms regulating platelet responses downstream of ADP receptors are not completely known.
The aim of the current study is to elucidate the role of PKC isoforms in regulating ADP-induced platelet functional responses in platelets.
We sought to delineate the mechanism of ADP-induced platelet responses by performing platelet aggregation (aggregometry), ATP secretion (luciferin-luciferase reaction) and thromboxane generation (ELISA kit measuring TxB2) in human or murine platelets by pre-incubating the platelets with control (DMSO) or inhibitors wherever mentioned. We also evaluated the role of PKCd to ADP-induced platelet responses by using murine platelets lacking PKCd.
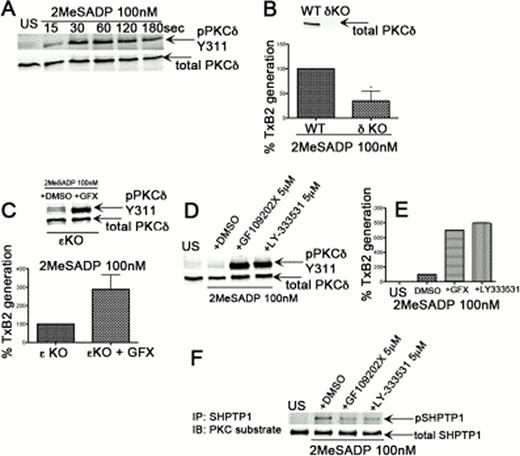
Murugappan et al have shown that PKCd was not activated downstream of ADP receptors based on the inability of ADP to cause threonine 507 phosphorylation on PKCd in platelets. However, studies from other labs have shown that PKCd can be activated when it is phosphorylated on its tyrosine residues. In the current study we show that, upon stimulation with 2MeSADP, PKCd is phosphorylated on tyrosine residue 311 in a time-dependent manner in platelets (Fig A). Also, ADP-induced thromboxane generation (Fig B) and ADP-induced thromboxane-mediated dense granule secretion were significantly inhibited in PKCd knockout murine platelets compared to those of wild type platelets. Similarly, thromboxane generation downstream of ADP receptors in human platelets pre-incubated with a PKCd inhibitor is significantly inhibited compared to control indicating a role for PKCd in mediating ADP-induced responses in platelets.
Bynagari et al have shown that ADP-induced thromboxane generation is potentiated in the presence of the pan-PKC inhibitor, GF 109203X and the isoform regulating this effect is PKCe. We observed that pre-incubation of PKCe knockout murine platelets with GF 109203X further potentiated ADP-induced thromboxane generation suggesting that there are other PKC isoforms negatively regulating ADP-induced thromboxane generation. We show that this potentiating effect of thromboxane generation with GF 109203X in WT or PKCe KO murine platelets correlate with an increase in the phosphorylation of Y311 on PKCd (Fig C) suggesting that ADP-induced thromboxane generation is regulated through PKCd Y311 phosphorylation.
Tyrosine phosphorylation on PKCd is mediated by Src family kinases (SFKs) as the phosphorylation is abolished with PP2, a SFK inhibitor and is only partially inhibited in Fyn knockout murine platelets suggesting that other SFKs also mediate this tyrosine phosphorylation.
Surprisingly, pre-incubation of platelets with LY-333531, a classical PKC isoform (a/b) inhibitor potentiated PKCd Y311 phosphorylation (Fig D) as well as thromboxane generation (Fig E) downstream of ADP receptors suggesting a role for classical PKCs. Also, platelets pre-incubated with LY-333531 showed a decrease in the phosphorylation of SHPTP-1 (Fig F), a tyrosine phosphatase, rendering it active. The active SHPTP-1 phosphatase may dephosphorylate and activate SFKs, which can now phosphorylate PKCd on Y311 in platelets.
In the current study, we report for the first time that the novel PKC isoform d is tyrosine phosphorylated downstream of ADP receptors through which it mediates ADP-induced thromboxane generation. We also show a novel role for classical PKC isoforms a/b in regulating tyrosine phosphorylation on novel isoform, PKCd possibly through the tyrosine phosphatase SHPTP-1 and Src family kinases in platelets.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal