Abstract
The erythropoietic effects of lenalidomide are cytokine dependent, suggesting that the erythroid hematologic improvement (HI-E) rate may be augmented by combined treatment (CT) with recombinant human erythropoietin (rhu-EPO) in myelodysplastic syndrome (MDS). In the present study, we explored the benefits of CT and the relationship between lenalidomide pharmacokinetics and hematologic toxicity in transfusion-dependent patients with low- to intermediate-1–risk MDS who failed prior rhu-EPO. In stage I, patients received 10 or 15 mg/d of lenalidomide monotherapy. At week 16, erythroid nonresponders (NRs) were eligible for CT with rhu-EPO 40 000 U/wk. Among 39 patients, HI-E response rate to monotherapy was 86% (6 of 7) in del(5q) and 25% (8 of 32) in non-del(5q) patients (10 mg, 17.7%; 15 mg, 33.3%). Twenty-three patients proceeded to CT, with 6 (26.0%) achieving HI-E. In 19 non-del(5q) patients, 4 (21.1%) showed HI-E. Mean baseline serum EPO in non-del(5q) patients was lower in monotherapy and CT responders than in NR (not statistically significant). Thrombocytopenia was significantly correlated with lenalidomide area under the plasma concentration-time curve (P = .0015), but severity of myelosuppression did not. The benefits of lenalidomide plus rhu-EPO are currently under investigation in a phase 3 Eastern Cooperative Oncology Group (ECOG)–sponsored intergroup study. This study is registered at www.clinicaltrials.gov as NCT00910858.
Introduction
Anemia and transfusion dependence remain the principal treatment challenges for patients with lower-risk myelodysplastic syndrome (MDS).1 In patients with low transfusion burden and endogenous erythropoietin (EPO) response, recombinant erythropoietic-stimulating agents (ESAs) have been shown to improve erythropoiesis.2 The immunomodulatory drug lenalidomide has EPO-promoting activity that is karyotype dependent. Lenalidomide suppresses the growth of MDS progenitors harboring a chromosome 5q deletion [del(5q)], giving rise to a high frequency of transfusion independence and cytogenetic response. In MDS patients with alternate karyotypes, lenalidomide promotes erythroid lineage competence and colony-forming capacity.3-5 Ebert et al showed that lenalidomide restores transcriptional response to EPO in MDS progenitors, suggesting that lenalidomide enhances cellular responsiveness to EPO stimulation.6 This dual mechanism of action may account for the differences in quality and rates of erythroid response, as well as the frequency and severity of myelosuppression, between del(5q) and non-del(5q) patients.7
We previously reported that lenalidomide improved erythropoiesis in MDS patients who had failed treatment with ESAs or had poor ESA response profiles.3,8 Given the capacity of lenalidomide to enhance the generation of EPO-responsive progenitors in vitro and in vivo,9 we hypothesized that combined treatment with epoetin alfa in patients who had failed ESA treatment should augment the erythropoietic response in those patients who experience suboptimal improvement with lenalidomide monotherapy. In the present study, we report hematologic response rates to lenalidomide monotherapy and combined treatment with recombinant epoetin alfa, and provide the first data describing the relationship between lenalidomide pharmacokinetics and hematologic toxicity in patients with MDS.
Methods
Patients
The study protocol was approved by our institutional review board, and informed consent was obtained from all patients in accordance with the Declaration of Helsinki (www.clinicaltrials.gov identifier NCT00910858). Eligible patients had a confirmed diagnosis of MDS with low- or intermediate-1–risk disease according to International Prognostic Scoring System (IPSS) criteria.10 Patients had symptomatic anemia (hemoglobin < 9.0 g/dL) or RBC transfusion-dependent anemia, defined as having received ≥ 4 transfusions of RBCs within 56 days of randomization. Patients either had no response to treatment with recombinant EPO (≥ 40 000 U/wk × > 6) or had an endogenous serum EPO level > 500 mU/mL. Other inclusion criteria included age ≥ 18 years and Eastern Cooperative Oncology Group (ECOG) performance status 0-2; women of childbearing potential had to agree to use 2 reliable forms of contraception simultaneously or to practice complete sexual abstinence. The study excluded patients with an absolute neutrophil count < 500 cells/μL, platelet count < 50 000/μL, serum creatinine more than the upper limit of normal, serum aspartate transaminase or alanine aminotransferase > 2 times the upper limit of normal, or total bilirubin > 2.0 mg/dL. Patients with secondary MDS, proliferative chronic myelomonocytic leukemia (WBC ≥ 12 000/μL), known HIV history, and prior history of malignancy other than MDS (except nonmelanoma skin cancer or carcinoma in situ of the cervix or breast) were also excluded unless the patient was free of disease for ≥ 3 years. Prior use of lenalidomide and use of cytotoxic chemotherapeutic agents, EPO, or experimental agents within 28 days of the first day of study drug treatment were also not allowed. All analyses included patients who had taken at least 1 dose of study drug.
Trial design
This was a 3-stage, open-label, single-center study. The first stage was the pharmacokinetic (PK) phase of the study to characterize the PK profile of lenalidomide in patients with low-risk or intermediate-1–risk MDS. On day −7 after a single oral dose of lenalidomide (10 mg), blood was collected at 0, 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, 12, and 24 hours, and parameters such as maximal plasma concentration (Cmax), time of maximal concentration (Tmax), area under the plasma concentration-time curve (AUC), clearance, volume of distribution, terminal elimination rate constant, and apparent terminal half-life were determined using noncompartmental methods.
The second stage of the study was lenalidomide monotherapy on a continuous daily treatment schedule. The first 25 patients received an oral dose of 10 mg daily; a second cohort of 15 patients received 15 mg daily. For patients who received 10 mg, a day-14, 5-hour–limited steady-state PK evaluation was performed. After completion of 16 weeks of lenalidomide monotherapy, erythroid nonresponders or those erythroid responders who experienced relapse of anemia at any time point after 16 weeks were offered participation in the third stage of the study: the combined treatment phase. For stage III, patients continued treatment with the same dose of lenalidomide in conjunction with recombinant human epoetin alfa (rhu-EPO) at a dose of 40 000 U/wk by subcutaneous injection.
During the screening process, transfusion history, complete blood counts (CBCs), serum chemistries, pregnancy testing, thyroid function tests, serum EPO level, BM aspirates and biopsies, and standard BM metaphase cytogenetics were assessed. Screening BM aspirate/biopsy and cytogenetic studies were performed within 56 days of study treatment. A baseline CBC was required within 7 days of the initial exposure to lenalidomide. All other eligibility criteria were to be assessed within 28 days of the initial exposure to lenalidomide.
CBCs were monitored weekly during the first 8 weeks of study treatment and every 2 weeks thereafter. A BM aspirate and biopsy were repeated within 72 hours of documentation of the first occurrence of grade 4 neutropenia or thrombocytopenia. BM aspiration/biopsy and cytogenetic analyses for response evaluation were assessed after 16 weeks of therapy. Patients with a response continued lenalidomide until disease progression, treatment failure, or development of dose-limiting adverse events. Patients with hematologic improvement who did not qualify as a protocol-defined responder after 16 weeks received 8 additional weeks of lenalidomide monotherapy before the final assessment of response. Patients were assessed for erythroid response after 8 weeks of combination therapy, with responders continuing treatment until failure or limiting toxicity. All patients were to complete off-study evaluations including a BM aspirate and biopsy with cytogenetic analysis and laboratory studies.
Assessment of response and toxicity
Hematologic response was assessed according to International Working Group (IWG 2000) criteria.11 A major erythroid response was defined as freedom from transfusion or an increase in the hemoglobin level of > 2 g/dL in patients with transfusion-independent anemia that was sustained for > 56 consecutive days. A minor response was defined as at least a 50% reduction in transfusions or a sustained elevation in the hemoglobin level of 1-2 g/dL. Because new IWG response criteria were proposed in 2006, the revised criteria for erythroid response were also applied as post hoc analysis. The IWG 2006 criteria omit minor erythroid responses, thereby accepting only clinically significant or major erythroid improvements. Adverse events were graded with the use of the Common Toxicity Criteria Version 3.0 of the National Cancer Institute.
Statistical analysis
Descriptive statistics were used for baseline characteristics. Estimate of the erythroid response rate in the monotherapy stage was an exploratory component and was not powered to provide sufficient confidence of the true response rate. The response rate and safety data were summarized when all patients reached at least 6 months of follow-up or on withdrawal from the study before 6 months. To assess duration of response, patients were followed until the next need for transfusion, disease progression, or withdrawal from the study for other reasons. The primary objective of the combined treatment phase was to estimate the erythroid response rate to lenalidomide plus rhu-EPO in patients who failed to respond during the monotherapy phase. This phase of the study was a feasibility and exploratory component and was not powered to provide sufficient confidence of the true response rate. The combined-treatment phase provided an estimate of patient acceptance of combined investigational treatment after prolonged monotherapy and treatment tolerance.
Two-sided t tests for means were used to compare lenalidomide exposure (AUC5 and Cmax) between those with and without neutropenia and/or thrombocytopenia. The mean serum EPO levels at start of the monotherapy phase and combined phase in erythroid responders and nonresponders were compared. The duration of transfusion independence was calculated from the date of the last RBC transfusion to the resumption of transfusion. The duration of major responses in transfusion-independent patients was recorded from the initial date of the sustained elevation in hemoglobin levels of > 2 g/dL. Because of the early stage of the study, all patients who received at least 1 dose of study drug (the safety population) were included in both the efficacy and safety analyses.
Results
Between January 2005 and October 2007, 40 patients were enrolled and 39 received at least 1 dose of study drug; all patients had failed prior ESA treatment and were RBC transfusion dependent. The mean age was 71 years (range, 45-85), and the mean transfusion burden was 4 units (range, 2-10) every 4 weeks. Among the 39 patients treated in the lenalidomide monotherapy phase, 23 patients continued into the combination phase with lenalidomide and epoetin alfa. Baseline characteristics of patients are summarized in Table 1. No differences were noted between patients on monotherapy and the subset of patients who continued to the combination therapy. Of 39 patients, 7 patients had del(5q).
Baseline characteristics for patients enrolled on lenalidomide monotherapy and lenalidomide and (rhu-EPO) combined therapy
| . | Monotherapy (n = 39) . | Combination therapy (n = 23) . |
|---|---|---|
| Mean age, y | 70.8 | 70.1 |
| Sex | ||
| Male | 28 (71.8%) | 18 (78.3%) |
| Female | 11 (28.2%) | 5 (21.7%) |
| ECOG performance status | ||
| 0 | 7 (17.9%) | 6 (26.1%) |
| 1 | 31 (79.5) | 17 (73.9%) |
| 2 | 1 (2.6%) | 0 |
| Median duration of MDS, y (range) | 1.9 (0.4-13.3) | 3.2 |
| Median RBC transfusion burden for 4 weeks, n (range) | 4 (2.0-10.0) | |
| MDS FAB subtype, n (%) | ||
| RA | 11 (28%) | 7 (30%) |
| RARS | 15 (38.5%) | 7 (30%) |
| RCMD | 4 (10.0%) | 3 (13%) |
| RAEB | 8 (21%) | 5 (22%) |
| CMML | 1 (2.5%) | 1 (4.3%) |
| Cytogenetics, n (%) | ||
| Non-del(5q) | 32 (82%) | 19 (83%) |
| del(5q) | 7 (18%) | 4 (17%) |
| IPSS, n (%) | ||
| Low risk | 18 (46.2%) | 11 (47.8%) |
| Intermediate-1 risk | 21 (53.8%) | 12 (52.2%) |
| Serum EPO level | ||
| Mean, IU (SD) | 955 (1652) | 600 (921) |
| Patients > 500 IU, n (%) | 15/36 (39%) | 7/23 (30%) |
| . | Monotherapy (n = 39) . | Combination therapy (n = 23) . |
|---|---|---|
| Mean age, y | 70.8 | 70.1 |
| Sex | ||
| Male | 28 (71.8%) | 18 (78.3%) |
| Female | 11 (28.2%) | 5 (21.7%) |
| ECOG performance status | ||
| 0 | 7 (17.9%) | 6 (26.1%) |
| 1 | 31 (79.5) | 17 (73.9%) |
| 2 | 1 (2.6%) | 0 |
| Median duration of MDS, y (range) | 1.9 (0.4-13.3) | 3.2 |
| Median RBC transfusion burden for 4 weeks, n (range) | 4 (2.0-10.0) | |
| MDS FAB subtype, n (%) | ||
| RA | 11 (28%) | 7 (30%) |
| RARS | 15 (38.5%) | 7 (30%) |
| RCMD | 4 (10.0%) | 3 (13%) |
| RAEB | 8 (21%) | 5 (22%) |
| CMML | 1 (2.5%) | 1 (4.3%) |
| Cytogenetics, n (%) | ||
| Non-del(5q) | 32 (82%) | 19 (83%) |
| del(5q) | 7 (18%) | 4 (17%) |
| IPSS, n (%) | ||
| Low risk | 18 (46.2%) | 11 (47.8%) |
| Intermediate-1 risk | 21 (53.8%) | 12 (52.2%) |
| Serum EPO level | ||
| Mean, IU (SD) | 955 (1652) | 600 (921) |
| Patients > 500 IU, n (%) | 15/36 (39%) | 7/23 (30%) |
CMML indicates chronic myelomonocytic leukemia; FAB, French-American-British system; RA, refractory anemia; RAEB, refractory anemia with excess blasts; RARS, refractory anemia with ring sideroblasts; and CMD, refractory cytopenia with multilineage dysplasia.
Relationship between plasma lenalidomide exposure and hematologic toxicities
Hematologic data were obtained from 24 patients who received the lenalidomide 10-mg dose during the first cycle. The highest grade of neutropenia/thrombocytopenia observed during this period (days 1-29) for each patient was used for analysis. Lenalidomide AUC5 and Cmax were determined on day 14 of the first cycle (at steady state).
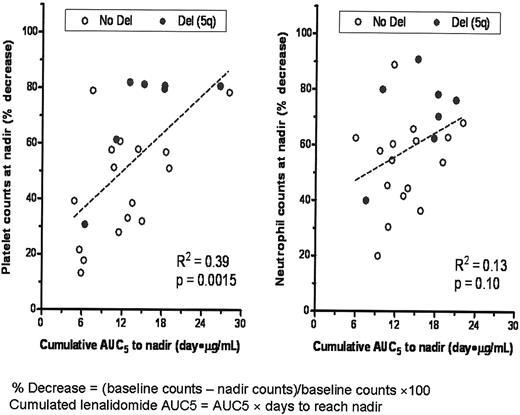
The correlation between treatment-emergent decline in platelet or neutrophil counts and cumulative lenalidomide AUC5 was assessed by linear regression in patients who had both evaluable hematologic and PK data in cycle 1 (Figure 1). Similar relationships were observed with cumulative Cmax, but to a lesser degree (data not shown). The magnitude of decrease in the platelet count from baseline was significantly correlated with cumulative lenalidomide plasma exposure (P = .0015). Severity of neutropenia tended to be greater with higher cumulative lenalidomide exposure, but this relationship was not statistically significant (P = .10). Results for mean AUC5 (ng/h/mL) and Cmax (ng/mL) were 635 ± 182 and 217 ± 75, respectively, in patients with grade 3/4 thrombocytopenia or neutropenia compared with 485 ± 196 and 151 ± 58, respectively, in patients without grade 3/4 thrombocytopenia or neutropenia (thrombocytopenia, P = .041; neutropenia, P = .025).The interval to development of thrombocytopenia and neutropenia was shorter in MDS patients with higher lenalidomide AUC than in those with lower AUC5 (Table 2). MDS patients with del(5q) had a greater decrease in platelet and neutrophil counts at nadir than those without the deletion (P < .05). This difference was not associated with level of lenalidomide exposure (Table 3).
Linear correlation between cumulative lenalidomide exposure and thrombocytopenia and neutropenia.
Linear correlation between cumulative lenalidomide exposure and thrombocytopenia and neutropenia.
Relationship between time to 50% reduction in neutrophil or platelet counts and steady-state lenalidomide exposure
| Time to ≥ 50% reduction from baseline . | Neutrophils . | Platelets . | ||||
|---|---|---|---|---|---|---|
| n . | Cmax, ng/mL . | AUC5, h/ng/mL . | n . | Cmax, ng/mL . | AUC5, h/ng/mL . | |
| < 28 days | 12 | 218 ± 45 | 683 ± 187 | 11 | 208 ± 49 | 656 ± 201 |
| ≥ 28 days | 10 | 162 ± 96 | 449 ± 98 | 9 | 169 ± 105 | 460 ± 121 |
| P | .016 | .0008 | .076 | .011 | ||
| Time to ≥ 50% reduction from baseline . | Neutrophils . | Platelets . | ||||
|---|---|---|---|---|---|---|
| n . | Cmax, ng/mL . | AUC5, h/ng/mL . | n . | Cmax, ng/mL . | AUC5, h/ng/mL . | |
| < 28 days | 12 | 218 ± 45 | 683 ± 187 | 11 | 208 ± 49 | 656 ± 201 |
| ≥ 28 days | 10 | 162 ± 96 | 449 ± 98 | 9 | 169 ± 105 | 460 ± 121 |
| P | .016 | .0008 | .076 | .011 | ||
Hematological data were obtained from 22 patients who received lenalidomide 10 mg daily for up to 28 days during the first cycle. Lenalidomide AUC5 and Cmax were determined on day 14 of the first cycle (at steady state).
Significantly different between the 2 groups (< 28 days or ≥ 28 days to 50% or more reduction from baseline) as shown by 2-sided t test after log-transformation of the exposure data.
Comparison of hematological toxicity and lenalidomide exposure between patients with and without the del(5q) abnormality
| . | del(5q) abnormality . | P . | |
|---|---|---|---|
| No (n = 17) . | Yes (n = 7) . | ||
| Myelosuppression | |||
| Grade 3 or 4 neutropenia | 47.1% | 100% | .022* |
| Grade 3 or 4 thrombocytopenia | 23.5% | 57.1% | .167 |
| Grade 3 or 4 neutropenia and/or thrombocytopenia | 58.8% | 100% | .065 |
| Grade 3 or 4 neutropenia and thrombocytopenia | 11.8% | 57.1% | .038* |
| Decrease in neutrophil counts at nadir | 53.4% ± 16.6% | 71.2% ± 16.3% | .028* |
| Decrease in platelet counts at nadir | 44.7% ± 20.1% | 70.9% ± 19.2% | .008* |
| Lenalidomide exposure | |||
| Lenalidomide Cmax at steady state, ng/mL | 193 ± 84 | 211 ± 51 | .393 |
| Lenalidomide AUC5 at steady state, ng/h/mL | 568 ± 201 | 647 ± 179 | .291 |
| Lenalidomide AUCcum to neutrophil nadir, μg/h/mL | 13.5 ± 4.3 | 15.5 ± 4.9 | .431 |
| Lenalidomide AUCcum to platelet nadir, μg/h/mL | 12.3 ± 6.3 | 15.6 ± 6.4 | .235 |
| . | del(5q) abnormality . | P . | |
|---|---|---|---|
| No (n = 17) . | Yes (n = 7) . | ||
| Myelosuppression | |||
| Grade 3 or 4 neutropenia | 47.1% | 100% | .022* |
| Grade 3 or 4 thrombocytopenia | 23.5% | 57.1% | .167 |
| Grade 3 or 4 neutropenia and/or thrombocytopenia | 58.8% | 100% | .065 |
| Grade 3 or 4 neutropenia and thrombocytopenia | 11.8% | 57.1% | .038* |
| Decrease in neutrophil counts at nadir | 53.4% ± 16.6% | 71.2% ± 16.3% | .028* |
| Decrease in platelet counts at nadir | 44.7% ± 20.1% | 70.9% ± 19.2% | .008* |
| Lenalidomide exposure | |||
| Lenalidomide Cmax at steady state, ng/mL | 193 ± 84 | 211 ± 51 | .393 |
| Lenalidomide AUC5 at steady state, ng/h/mL | 568 ± 201 | 647 ± 179 | .291 |
| Lenalidomide AUCcum to neutrophil nadir, μg/h/mL | 13.5 ± 4.3 | 15.5 ± 4.9 | .431 |
| Lenalidomide AUCcum to platelet nadir, μg/h/mL | 12.3 ± 6.3 | 15.6 ± 6.4 | .235 |
Data were obtained from 24 patients who received lenalidomide 10 mg daily for up to 28 days during the first cycle. Lenalidomide AUC5 and Cmax were determined on day 14 of the first cycle (at steady state). Cumulated lenalidomide AUC5 = AUC5 × days to reach nadir. A 2-sided Fisher exact test was used to compare percentage of patients with grade 3 or 4 neutropenia/thrombocytopenia between groups, and 2-sided t test was used to compare all other parameters between groups.
Significantly different between “no” and “yes” groups.
PK analyses showed no evidence of drug accumulation after 14 days of treatment. Lenalidomide AUC was highest in patients with reduced creatinine clearance (at 30-49 mL/min [n = 2]: 955 ± 49; at > 50 mL/min [n = 22]: 558 ± 166).
Erythroid responses
The overall rate of erythroid hematologic improvement (HI-E) to lenalidomide monotherapy in del(5q) patients was 86% (6 of 7 patients), all of which were major erythroid responses (MERs) according to IWG 2000 criteria and 86% had hematologic erythroid improvement (HI-E) according to IWG 2006 criteria. In non-del(5q) patients, the HI-E rate was 25.0% (8 of 32 patients) with MERs in 21.9% (7 patients) and minor erythroid responses in 3.1% (1 patient). By IWG criteria 2006, the HI-E rate was 19% (6 of 32).
Among 32 non-del(5q) patients, 17 patients received the 10-mg dose and 15 received the 15-mg dose of lenalidomide. The rate of HI-E was 17.7% (3 of 17) and the rate of MER was 11.8% (2 of 17) for patients receiving 10 mg, whereas the HI-E rate was 33.3% (5 of 15) and the MER rate was 33.3% (5/15) in patients receiving 15 mg of lenalidomide.
During the monotherapy phase, according to IWG 2000 criteria, the mean time to MER was 37 and 41 days in del(5q) and non-del(5q) patients, respectively. The mean duration of response in del(5q) patients was 666 days versus 338 days in non-del(5q) patients.
Twenty-three patients received combined treatment with lenalidomide and epoetin alfa, including 19 of the 32 non-del(5q) patients and 4 of the 7 del(5q) patients. Among the 19 patients with non-del(5q), all 4 responding patients (21.1%) had a MER, 3 of whom did not respond to lenalidomide monotherapy and 1 who had a minor erythroid response. The mean time to MER in non-del(5q) patients receiving combination therapy was 187 days from start of monotherapy, and the mean duration of response was 1056 days from start of combination therapy, with 2 ongoing responses. The response rate to combined therapy in non-del 5 q patients by IWG 2006 criteria was 16% (3 of 19). Among the 4 patients with del(5q), 2 had HI-E with combined therapy, 1 showed MER, and 1 showed minor response. Mean time to response from initiating monotherapy was 932 and 557 days, respectively, reflecting the later addition of ESA at time of treatment failure as a result of the longer duration of response to monotherapy. The mean duration of response was 211 days. The HI-E by IWG 2006 criteria among del(5q) MDS patients receiving combined therapy after lenalidomide monotherapy failure was 25% (1 of 4). Table 4 summarizes all response rates.
Erythroid response rates
| . | n . | IWG 2000 HI-E . | IWG 2000-MER . | IWG 2006 HI-E . |
|---|---|---|---|---|
| Lenalidomide monotherapy (N = 39) | ||||
| Del(5q) | 7 | 6 (85.7%) | 6 (85.7%) | 6 (85.7%) |
| Non-del(5q) | 32 | 8 (25.0%) | 7 (21.9%) | 6 (19%) |
| 10 mg | 17 | 3 (17.6%) | 2 (11.8%) | |
| 15 mg | 15 | 5 (33.3%) | 5 (33.3%) | |
| Combination therapy | 23 | 6 (26.0%) | 5 (21.7%) | |
| Del(5q) | 4 | 2 (50.0%) | 1 (25.0%) | 1 (25.0%) |
| Non-del(5q) | 19 | 4 (21.1%) | 4 (21.1%) | 3 (16%) |
| 10 mg | 9 | 2 (22.2%) | 2 (22.2%) | |
| 15 mg | 10 | 2 (20.0%) | 2 (20.0%) |
| . | n . | IWG 2000 HI-E . | IWG 2000-MER . | IWG 2006 HI-E . |
|---|---|---|---|---|
| Lenalidomide monotherapy (N = 39) | ||||
| Del(5q) | 7 | 6 (85.7%) | 6 (85.7%) | 6 (85.7%) |
| Non-del(5q) | 32 | 8 (25.0%) | 7 (21.9%) | 6 (19%) |
| 10 mg | 17 | 3 (17.6%) | 2 (11.8%) | |
| 15 mg | 15 | 5 (33.3%) | 5 (33.3%) | |
| Combination therapy | 23 | 6 (26.0%) | 5 (21.7%) | |
| Del(5q) | 4 | 2 (50.0%) | 1 (25.0%) | 1 (25.0%) |
| Non-del(5q) | 19 | 4 (21.1%) | 4 (21.1%) | 3 (16%) |
| 10 mg | 9 | 2 (22.2%) | 2 (22.2%) | |
| 15 mg | 10 | 2 (20.0%) | 2 (20.0%) |
Analysis of the relationship between serum EPO (sEPO) level and treatment response in non-del(5q) patients showed that, for patients receiving lenalidomide monotherapy (n = 32), the mean sEPO at baseline in erythroid responders was 255 mU/mL (SD = 283; n = 8) compared with 870 mU/mL (SD = 1298) in nonresponders (n = 24; P = nonsignificant). For non-del(5q) MDS patients who failed to respond to lenalidomide and proceeded to combined treatment with epoetin alfa (n = 19), the mean sEPO in responders at the start of combination therapy was 318 mU/mL (SD 443; n = 4) compared with 732 mU/mL in nonresponders (SD = 1107; n = 15; P = nonsignificant).
Adverse events
Treatment-related adverse events were similar to those reported with lenalidomide in prior studies. Table 5 summarizes grade 3/4 treatment-related adverse events. No differences in toxicity profile were observed in the combination therapy versus monotherapy groups. The only venous thromboembolic (VTE) event that occurred was a deep venous thrombosis in a non-del(5q) patient undergoing monotherapy. There were no VTEs reported in patients receiving combination therapy.
Incidence of adverse events
| Event, n (%) . | Non-del(5q) (n = 32) . | Del(5q) (n = 7) . | ||
|---|---|---|---|---|
| Grade 3 . | Grade 4 . | Grade 3 . | Grade 4 . | |
| Thrombocytopenia | 9 (28.1) | 10 (31.3) | 3 (42.9) | 2 (28.6) |
| Neutropenia | 8 (25.0) | 15 (46.9) | 2 (28.6) | 5 (71.4) |
| Anemia | 2 (6.3) | 0 (0.0) | 4 (57.1) | 0 (0.0) |
| Diarrhea | 1 (3.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Constipation | 1 (3.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Nausea | 1 (3.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Vomiting | 1 (3.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Pruritus and rash | 5 (15.6) | 0 (0.0) | 1 (14.3) | 0 (0.0) |
| Muscle cramps | 2 (6.3) | 0 (0.0) | 2 (28.6) | 0 (0.0) |
| Fatigue | 2 (6.3) | 0 (0.0) | 1 (14.3) | 0 (0.0) |
| Infections | 1 (3.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Deep venous thrombosis | 1 (3.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Event, n (%) . | Non-del(5q) (n = 32) . | Del(5q) (n = 7) . | ||
|---|---|---|---|---|
| Grade 3 . | Grade 4 . | Grade 3 . | Grade 4 . | |
| Thrombocytopenia | 9 (28.1) | 10 (31.3) | 3 (42.9) | 2 (28.6) |
| Neutropenia | 8 (25.0) | 15 (46.9) | 2 (28.6) | 5 (71.4) |
| Anemia | 2 (6.3) | 0 (0.0) | 4 (57.1) | 0 (0.0) |
| Diarrhea | 1 (3.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Constipation | 1 (3.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Nausea | 1 (3.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Vomiting | 1 (3.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Pruritus and rash | 5 (15.6) | 0 (0.0) | 1 (14.3) | 0 (0.0) |
| Muscle cramps | 2 (6.3) | 0 (0.0) | 2 (28.6) | 0 (0.0) |
| Fatigue | 2 (6.3) | 0 (0.0) | 1 (14.3) | 0 (0.0) |
| Infections | 1 (3.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Deep venous thrombosis | 1 (3.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Twenty-one (65.6%) of the 32 patients with non-del(5q) had at least 1 dose interruption, primarily because of thrombocytopenia or neutropenia. The median time to first dose interruption was 27 days, and the median duration of dose interruption was 9 days. Six (85.7%) patients with del(5q) had at least 1 dose interruption because of myelosuppression. The median time to first dose interruption was 28 days, and the median duration of dose interruption was 17.5 days. Twenty (62.5%) of the 32 patients in the non-del(5q) cohort had at least 1 dose reduction, and 6 patients (85.7%) in the del(5q) cohort had 1 or more dose reductions. Six non-del(5q) patients had to discontinue treatment because of adverse events (4 had thrombocytopenia, 1 had hemolytic anemia, and 1 had rash).
No significant changes in BM cellularity or the myeloid-to-erythroid ratio in non-del5q patients (n = 10; P = .566) were observed at absolute neutrophil count nadir time.
Discussion
Lenalidomide was approved by the US Food and Drug Administration for the treatment of transfusion-dependent, low-, and intermediate-1–risk MDS patients with del(5q) based on a high rate of durable transfusion independence and corresponding cytogenetic response.3,12 A companion trial of identical design performed in patients without del(5q) (MDS-002) yielded a much lower rate of transfusion response (43%); 26% achieved transfusion independence with a 3.2 g/dL median hemoglobin rise and median duration of response of < 1 year.4 These results are consistent with those of the original dose-finding study.8 These findings indicate that, as a single agent, lenalidomide has modest erythroid-promoting activity in non-del(5q) MDS patients, but represents an alternative for patients unresponsive to an ESA.
The frequency of erythroid response to lenalidomide monotherapy in non-del(5q) MDS patients in this trial was lower than that reported in prior studies (25%). Although only 15 patients were treated with the 15-mg dose of lenalidomide, the erythroid response was higher (33%) than the cohort treated with 10 mg (18%), raising the possibility of dose dependence. Nevertheless, in this heavily transfusion-dependent population that was previously resistant to rhu-EPO, the addition of epoetin alfa to lenalidomide treatment yielded an erythroid response rate of 26% in lenalidomide nonresponders, with 22% of patients achieving transfusion independence, thereby increasing the overall response rate to 51%. Although we cannot exclude the possibility that hematologic responses to combined therapy represent a delayed response to lenalidomide, prior studies have shown that the majority of patients respond to lenalidomide within 2-4 cycles, indicating that the responses that we observed reflect the benefit of the addition of epoetin alfa. Indeed, these findings confirm and validate in vitro study results suggesting that lenalidomide sensitizes MDS progenitors to the stimulatory effects of EPO and indicate that lenalidomide can allow patients to overcome clinical resistance to ESAs.13 Although the number of patients with lenalidomide secondary failures who were treated is small, the absence of response to combined treatment in this population is not surprising and remains consistent with the presumed ligand dependence of the drug's erythropoietic effects. We found no significant difference in mean serum endogenous EPO levels between responders and nonresponders to either monotherapy or combined therapy. Given the exploratory nature of this study and the sequential trial design, firm conclusions regarding potential benefits of combined treatment must await the results of an ongoing phase 3, ECOG-sponsored intergroup study comparing major erythroid response rates to treatment with lenalidomide to combined treatment with epoetin alfa in patients who failed to respond to ESAs (www.clinicaltrials.gov identifier NCT00843882).
The results of the present study provide preliminary evidence that combined treatment with lenalidomide plus epoetin alfa is safe in patients with MDS. Adverse events in this study were similar to those reported in prior lenalidomide studies. Although an increased frequency of VTEs was observed in lenalidomide-treated patients with multiple myeloma receiving ESAs, no VTEs were reported in our patients who received combination therapy. The higher rate of VTEs in the myeloma population may be related to the inclusion of corticosteroids and/or the concomitant use of traditional cytotoxic antineoplastics.
This is the first trial to examine the relationship between systemic drug exposure and treatment-related toxicities in MDS patients. We found that the magnitude of treatment-related thrombocytopenia was directly correlated with cumulative lenalidomide plasma exposure (P = .0015), as was the interval to development of thrombocytopenia (P = .011). Although the severity of neutropenia was greater with higher lenalidomide AUC, it did not reach statistical significance (P = .10); however, the interval to the development of neutropenia was correlated with cumulative lenalidomide exposure (P = .0008). The results of the present study provide the first evidence that differences in drug elimination may underlie the wide variance in severity and time to myelosuppression with lenalidomide treatment in patients with MDS.
Presented in part at the 2008 Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 30-June 3, 2008.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Rasa Hamilton (Moffitt Cancer Center) and Lena Shapiro (Celgene Corporation) for assistance with the preparation of this manuscript.
This trial was funded in part by Celgene Corporation.
Authorship
Contribution: R.S.K., J.E.L., A.S.S., N.C., J.P., R.L., H.I.S., and A.F.L. collected the data, wrote the manuscript, and approved the final version of the manuscript; A.S.S., N.C., R.L., and A.F.L. conceived and designed the study; R.S.K., J.E.L., A.S.S., N.C., R.L., and A.F.L. analyzed and interpreted the data; and J.E.L., H.I.S., and A.F.L. provided study materials or patients.
Conflict-of-interest disclosure: R.S.K. received research funding from and was a member of a speakers bureau for Celgene; J.E.L. received research funding and served as a consultant for Celgene; A.S.S. is the director of biostatistics for and owns stock in Celgene; N.C. is principal scientist for translational development and owns stock in Celgene; and A.F.L. received honoraria and research funding from Celgene. The remaining authors declare no competing financial interests.
Correspondence: Rami Komrokji, MD, Moffitt Cancer Center and Research Institute, 12902 Magnolia Dr, Tampa, FL 33612; e-mail: rami.komrokji@moffitt.org.