In this issue of Blood, Hassan et al have turned the spotlight on hematopoietic stem cell transplantation (HCT) of adenosine deaminase (ADA)–deficient severe combined immunodeficiency (SCID).1 They opened up the curtain of beliefs on this therapy that enables facts to be separated from fiction.
Hassan et al show what common beliefs concerning stem cell immunoreconstitution of ADA-SCID are true or not. To be helpful to the expectations of parents of such children, they also raise the critical issue of quality of life and educational and employment accomplishments as important goals not being sufficiently assessed.2,3 The results of this study are powerful and persuasive, as it is the only definitive analytical study of a large number of ADA-SCID children given HCT. ADA enzyme replacement and ADA gene therapy are also considered alternate therapies. The authors point out that the ADA deficiency affects many cells and the remedy for the immune cell lineages may not apply to other cell types, such as the central nervous system and respiratory system.
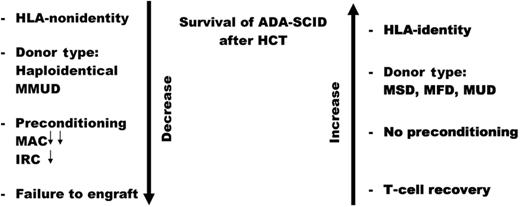
One hundred six ADA-SCID patients were entered into this multicenter analysis of the outcomes of their HCT therapy. The most prominent risk factor for a good outcome is the lack of perfect HLA matching as noted by an earlier publication.4 In the present study, HCT of ADA-SCID patients with these donors yielded the following overall survival (OS) results: mismatched unrelated donors, 29%; haploidentical donors, 43%; matched unrelated donors, 67%; matched family donors, 83%; and matched sibling donors, 86%.1 These results confirm the common belief that HLA matching is the most important risk factor for OS for ADA-deficient SCID children treated with HCT (see figure).
Risk factors for survival of adenosine deaminase deficient (ADA) patients with severe combined immunodeficiency (SCID) treated with hematopoietic stem cell transplantation (HCT). MMUD indicates mismatched unrelated donor; MAC, myeloablative conditioning; IRC, intensity reduced conditioning; MSD, matched sibling donor; MFD, matched family donor; and MUD, matched unrelated donor. Double small arrows MAC indicate lower survival versus the single small arrow with IRC.
Risk factors for survival of adenosine deaminase deficient (ADA) patients with severe combined immunodeficiency (SCID) treated with hematopoietic stem cell transplantation (HCT). MMUD indicates mismatched unrelated donor; MAC, myeloablative conditioning; IRC, intensity reduced conditioning; MSD, matched sibling donor; MFD, matched family donor; and MUD, matched unrelated donor. Double small arrows MAC indicate lower survival versus the single small arrow with IRC.
This large study also addressed the controversial question of preconditioning of SCID children before HCT.5 Myeloablative preconditioning was shown to be a risk factor for OS (56%), whereas reduced-intensity conditioning displayed a trend toward improved survival (67%) but was not significantly different from unconditioned transplants (78%), whereas myoablative therapy was. We must remember that almost all unconditioned transplants had the benefit of HLA matching so the superior survival is not unexpected, and ADA-deficient cells may be more susceptible to toxic effects of preconditioning agents. Unconditioned haploidentical transplants fared much worse in a small cohort with 1 of 6 survivors. Thus, this report has something for proponents on both sides of the debate on whether or not to precondition SCID patients before transplantation.
This study refutes the belief that prior ADA enzyme therapy is a risk factor for OS of patient after HCT.6 Those patients who were treated with ADA enzyme did as well as those not receiving ADA enzyme at the time of HCT. Similarly, this study does not support previous findings that early transplantation enhances overall outcome of patients,7 although it seems intuitive that a prolonged pretransplant period in an unprotected environment would favor acquisition of a potentially fatal virus infection. The HCT approach for ADA-SCID needs to be balanced with the concept of gene therapy where spectacular results (100% survival) have been achieved by Aiuti et al.8
As an indication of the fundamental difference between ADA-SCID and other forms of SCID, the immunoreconstitution of B cells and ability to produce and secrete immunoglobulins and specific antibodies in evaluable immunoreconstituted ADA-deficient patients is striking. Some of these patients, while not showing donor B-cell engraftment, were still able to make normal humoral immune responses, suggesting T:B-cell cooperation even with HLA disparity.
The need for such definitive studies as the Hassan et al article grows more intense with the advent of universal screening for all forms of SCID.9,10 Seven states and 1 territory have adopted the T-cell receptor excision circle (TREC) test of newborns by DNA analysis of dried blood spots on a Guthrie card. Early results indicate a higher incidence of SCID (1 in 40 000-60 000 vs previous estimates of 1 in 100 000 live births). With more SCID infants coming to diagnosis, including ADA-SCID infants, the need for analyses such as the Hassan et al study becomes urgent.
We thank Hassan et al for their monumental effort in trying to sort out the successes and failures of HCT for ADA-SCID, and performing statistical analysis of their results to better guide physicians and patients in making informed choices of therapy for their special children.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal