Abstract
Fms-like tyrosine kinase 3 (FLT3) is a receptor tyrosine kinase with important roles in hematopoietic progenitor cell survival and proliferation. It is mutated in approximately one-third of AML patients, mostly by internal tandem duplications (ITDs). Adaptor protein Lnk is a negative regulator of hematopoietic cytokine signaling. In the present study, we show that Lnk interacts physically with both wild-type FLT3 (FLT3-WT) and FLT3-ITD through the SH2 domains. We have identified the tyrosine residues 572, 591, and 919 of FLT3 as phosphorylation sites involved in direct binding to Lnk. Lnk itself was tyrosine phosphorylated by both FLT3 ligand (FL)–activated FLT3-WT and constitutively activated FLT3-ITD. Both shRNA-mediated depletion and forced overexpression of Lnk demonstrated that activation signals emanating from both forms of FLT3 are under negative regulation by Lnk. Moreover, Lnk inhibited 32D cell proliferation driven by different FLT3 variants. Analysis of primary BM cells from Lnk-knockout mice showed that Lnk suppresses the expansion of FL-stimulated hematopoietic progenitors, including lymphoid-primed multipotent progenitors. The results of the present study show that through direct binding to FLT3, Lnk suppresses FLT3-WT/ITD–dependent signaling pathways involved in the proliferation of hematopoietic cells. Therefore, modulation of Lnk expression levels may provide a unique therapeutic approach for FLT3-ITD–associated hematopoietic disease.
Introduction
The production and lineage commitment of hematopoietic progenitor cells (HPCs) is controlled by the actions of a complex network of signaling pathways.1 Mutations and translocations of tyrosine kinases within these pathways lead to constitutive signaling and enhanced proliferation. Classic examples are BCR-ABL in chronic myeloid leukemia,2 JAK2 mutations in a group of myeloproliferative disorders,3 and Fms-like tyrosine kinase 3 (FLT3) and c-KIT mutations in AML.4
FLT3 belongs to a family of type III receptor tyrosine kinases4,5 that also includes PDGFRs, FMS, and c-KIT. The structure of these kinases is characterized by an extracellular domain consisting of 5 Ig-like domains, a single transmembrane region, a cytoplasmic juxtamembrane (JXM) domain, and a tyrosine kinase domain.6 FLT3 is expressed on hematopoietic progenitor cells and regulates early steps of HPC proliferation, survival, and differentiation. Mutations in the receptor, both in the form of internal tandem duplication (ITD) of the JXM domain and point mutations of the tyrosine kinase domain (TKD), result in constitutive activation. Compared with the ligand-activated wild-type FLT3 (FLT3-WT) receptor, oncogenic FLT3-ITD activates aberrant signaling and shows stronger transforming potential.5,7 The downstream signaling pathways elicited by constitutive FLT3 activation have not been fully elucidated, but the STAT5, MAPK, and PI3K/AKT pathways have all been shown to be involved.8–10 FLT3 mutations occur in approximately one-third of AML patients and are one of the most common alterations in AML.11 FLT3-ITD and TKD mutations are also detectable in myeloproliferative neoplasms (MPNs),12 and several animal studies indicate that expression of FLT3-ITD alone is sufficient to induce MPN.13–15
Lnk (also known as SH2B3) is expressed in HPCs and plays a critical role in cytokine signaling and hematopoiesis.16–18 Together with SH2-B (SH2B1) and APS (SH2B2), Lnk belongs to a family of adaptor proteins that modulate signaling of several cytokine and growth factor receptors.19–21 These family members share common structural domains, including a dimerization domain (DD) at the amino (N)–terminus, a pleckstrin homology (PH) domain in the center, and an Src homology 2 (SH2) domain near the carboxyl-terminus. Lnk negatively modulates several important cytokine-induced signaling pathways, including the SCF/c-KIT, erythropoietin/JAK2, and thrombopoietin (TPO)/MPL-JAK2 pathways.17,22–24 Lnk also binds and regulates MPL-W515L– and JAK2-V617F–activated forms in hematopoetic cells.25,26 Recently, Lnk mutations that result in partial loss of function have been identified in MPN patients, suggesting an important role of Lnk in the development of the disease.27,28
Previously, we and others have shown that Lnk interacts with the JXM domain of c-KIT.29 We also found that Lnk binds to PDGFRA, PDGFRB, and FMS,30,31 all of which share a similar sequence in this domain. The fact that FLT3 harbors a conserved JXM domain (Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) prompted us to investigate whether Lnk interacts with FLT3. In the present study, we identify FLT3-WT and FLT3-ITD as novel binding partners of Lnk.
Methods
Mice and cell culture
Lnk−/−129/Sv mice were generously provided by T. Pawson (Samuel Lunenfeld Research Institute, Toronto, ON). BM cells derived from 8- to 10-week-old Lnk+/+ and Lnk−/− mice were obtained by flushing femurs and tibias with the appropriate medium. 32D/FLT3-WT, 32D/FLT3-TKD, and 32D/FLT3-ITD cells were generously provided by C. H. Brandts (Johann Wolfgang Goethe University, Frankfurt, Germany). The study was approved by the Institutional Animal Care and Use Committee of Cedars-Sinai Medical Center. 32D/FLT3-TKD, 32D/FLT3-ITD, REH, EOL-1, and U937 cells were grown in RPMI medium supplemented with 10% FBS. 32D/FLT3-WT cells were grown in RPMI 1640 medium containing 10% FBS and 40 ng/mL of FLT3-ligand (FL). COS-1 and 293T cells were grown in DMEM with 10% FBS.
Colony and limiting-dilution assays
Either total BM cells or lineage-depleted (Lin−) BM cells (Lineage Cell Depletion Kit; Miltenyi Biotec) were plated into semisolid methylcellulose (M3234; StemCell Technologies) supplemented with 100 ng of IL6 and 100 ng of either SCF or FL (PeproTech) at various cell concentrations (103-105 cells/mL). Colonies were counted after 7-10 days. Limiting-dilution assays were performed on sorted lymphoid-primed multipotent progenitors (LMPPs) in buffered IMDM supplemented with 10% FBS, 2% glutamine, 100 ng of IL6, and 100 ng of FL. To sort LMPPs, BM cells were first enriched for Lin− cells (EasySep Mouse Hematopoietic Progenitor Cell Enrichment Kit; StemCell Technologies) and then separated into a Sca1+Kit+Flt3+Lin− fraction using a FACSAria cell sorter (BD Biosciences). Increasing cell numbers were directly deposited into 96-well plates (48 wells per cell number). Positive wells were scored after 1 week.
Affinity fishing of Lnk with immobilized peptides
Peptides corresponding to the tyrosine motifs of the FLT3 intracellular domain, either phosphorylated or not, were synthesized and immobilized on UltraLink beads (Thermo Scientific) according to the manufacturer's instructions. Next, 50 μL of a 1:1 slurry was incubated at 4°C for 1 hour with either Lnk-transfected COS-1 cell lysates or recombinant glutathione S-transferase (GST)–fusion proteins. After washing 3 times with lysis buffer, the peptide-bound proteins were processed for Western blotting. Peptide sequences corresponding to the tyrosine motifs of FLT3 are listed in supplemental Table 2.
In vitro expression and phosphorylation of FLT3 intracellular domains
GST-fused constructs were transformed into DH5α or TKX1 Escherichia coli cells (Agilent Technologies) for production of GST-fusion proteins. The TKX1 E coli contains a tyrosine kinase controlled by tryptophan promoter and can phosphorylate proteins expressed in this strain. Proteins expressed in E coli DH5α were used as an nonphosphorylated controls. Production of the GST-fusion proteins was induced using 0.05mM isopropyl β-D-thiogalactoside overnight at 22°C. For phosphorylation induction, TKX1 bacteria containing each construct were resuspended in M9 medium with 10 μg/mL of indole acrylic acids for 6 hours at 37°C. Harvesting and purification of phosphorylated and nonphosphorylated GST-fusion proteins were carried out according to the manufacturer's protocol (GE Healthcare).
Statistical analysis
Two-tailed Student t tests were used. Statistical significance was defined as P < .05. L-Calc was used to calculate the cloning frequency, the confidence interval, and the statistical significance of the ratio of proportions between the 2 genotypes in limiting dilution assays.
For further details on the materials and methods used in this study, please see supplemental Methods.
Results
Lnk binds to FLT3-WT and FLT3-ITD through its SH2 domain
Ligand-induced tyrosine phosphorylation of MPL,32 c-KIT,17 or c-FMS30 enables the physical interaction of Lnk with the receptor. To determine whether Lnk interacts with FLT3-WT in a phosphorylation-dependent fashion, we first performed coimmunoprecipitation (co-IP) with lysates of either untreated or FL-treated cells using anti-FLT3 Ab and showed that Lnk bound to endogenous FLT3-WT after stimulation with FL in human leukemia cells REH and EOL-1 (Figure 1A-B). We next examined the association between Lnk and FLT3-ITD, the most common form of FLT3 mutations in AML. Because several human FLT3-ITD+ leukemia cells endogenously express very low levels of Lnk (data not shown), we transfected Lnk into 32D/FLT3-ITD cells, which stably expressed FLT3-ITD. Because FLT3-ITD is constitutively phosphorylated and activated, cells were not stimulated with FL. As shown in Figure 1C, Lnk clearly formed a complex with FLT3-ITD.
Interactions of Lnk with FLT3-WT/ITD in hematopoetic cells. EOL-1 (A) or REH (B) cells were serum-starved for 16 hours (−) and treated with FL for 15 minutes (+). Cell lysates were subjected to pull-down by either anti-FLT3 Ab or normal rabbit Ig, and the precipitates were subjected to Western blotting analysis using Lnk Abs. Asterisk indicates the nonspecific bands and the heavy chain of Ig. Supernatant (SP) after immunoprecipitation from EOL-1, THP-1, and REH cells was included to show the position of endogenous Lnk. (C) 32D/FLT3-ITD cells were transfected with V5-tagged-Lnk. Lysates were precipitated with anti-V5 Ab, and the precipitates were subjected to Western blotting analysis using either FLT3 or V5 Abs.
Interactions of Lnk with FLT3-WT/ITD in hematopoetic cells. EOL-1 (A) or REH (B) cells were serum-starved for 16 hours (−) and treated with FL for 15 minutes (+). Cell lysates were subjected to pull-down by either anti-FLT3 Ab or normal rabbit Ig, and the precipitates were subjected to Western blotting analysis using Lnk Abs. Asterisk indicates the nonspecific bands and the heavy chain of Ig. Supernatant (SP) after immunoprecipitation from EOL-1, THP-1, and REH cells was included to show the position of endogenous Lnk. (C) 32D/FLT3-ITD cells were transfected with V5-tagged-Lnk. Lysates were precipitated with anti-V5 Ab, and the precipitates were subjected to Western blotting analysis using either FLT3 or V5 Abs.
To determine which domain of Lnk is involved in the interaction, several mutated or deleted Lnk constructs were expressed together with FLT3-WT/ITD in 293T cells. We found that the Lnk SH2 domain was the major domain responsible for the interaction, because SH2-mutated (R392E; RE) or deleted (δSH2) forms of Lnk were not able to interact with FLT3-ITD, bound much more weakly to FLT3-WT, and did not respond to FL stimulation (Figure 2). The Lnk E208Q mutant (EQ) found in MPN patients27,28 was also shown to bind to FLT3-WT/ITD (Figure 2B and D). A co-IP assay in the opposite direction confirmed the association between Lnk and FLT3-WT/ITD. After stimulation with FL, only Lnk, not the RE mutant bound to tyrosine-phosphorylated FLT3-WT, became tyrosine phosphorylated (Figure 2C). Similar to the results obtained with FLT3-WT, Lnk but not the RE mutant was tyrosine phosphorylated in the present of FLT3-ITD (Figure 2F). These results indicate that activated FLT3-WT/ITD binds to and then phosphorylates Lnk, and that this process requires a functional SH2 domain of Lnk.
Lnk binds to FLT3-WT and FLT3-ITD through the SH2 domain. 293T cells were co-transfected with FLT3-WT/ FLT3-ITD and either empty vector (EV) or various V5-tagged Lnk constructs. Cells were serum starved for 16 hours (−) and treated with FL for 15 minutes (+, when indicated). Lysates were precipitated with either anti-FLT3 (A,B,D) or anti-V5 Abs (C,E,F), and the precipitates were subjected to Western blotting analysis using the indicated Abs. (G) Lysates from either U937 or 32D/FLT3-ITD cells were incubated with GST, GST-Lnk-SH2 (GST-SH2), or GST-Lnk-PH (GST-PH) fusion proteins. After pull-down, protein complexes were analyzed by Western blotting with FLT3 Ab. Equal loading of the GST fusion protein was shown by Coomassie staining of the gel (bottom).
Lnk binds to FLT3-WT and FLT3-ITD through the SH2 domain. 293T cells were co-transfected with FLT3-WT/ FLT3-ITD and either empty vector (EV) or various V5-tagged Lnk constructs. Cells were serum starved for 16 hours (−) and treated with FL for 15 minutes (+, when indicated). Lysates were precipitated with either anti-FLT3 (A,B,D) or anti-V5 Abs (C,E,F), and the precipitates were subjected to Western blotting analysis using the indicated Abs. (G) Lysates from either U937 or 32D/FLT3-ITD cells were incubated with GST, GST-Lnk-SH2 (GST-SH2), or GST-Lnk-PH (GST-PH) fusion proteins. After pull-down, protein complexes were analyzed by Western blotting with FLT3 Ab. Equal loading of the GST fusion protein was shown by Coomassie staining of the gel (bottom).
The interactions were further verified with a GST pull-down assay in both 32D/FLT3-ITD cells and U937 cells that express endogenous FLT3-WT. We confirmed that SH2, but not the PH domain of Lnk, bound to FLT3-WT/ITD (Figure 2G).
Lnk binds directly to phosphorylated Tyr572, Tyr591, and Tyr919 of FLT3
Affinity fishing with immobilized peptides was performed to map the specific residues in FLT3 that interact with Lnk. Peptides corresponding to all of the known/predicted tyrosine motifs of the FLT3 intracellular domain, either phosphorylated or not, were incubated with Lnk purified from COS-1 cells. Motifs containing Tyr572, Tyr591, and Tyr919 were found to bind Lnk in a phosphodependent manner, with Tyr919 showing the strongest binding activity (Figure 3B-C). Furthermore, GST-fused Lnk SH2 domains associated with peptides containing these 3 tyrosine residues, indicating direct interactions between them (Figure 3D-E). In accordance with the GST pull-down results (Figure 2G), the Lnk PH domain did not exhibit any binding capacity.
Tyr572, Tyr591, and Tyr919 residues of FLT3 were responsible for phosphorylation-dependent binding to Lnk. (A) Diagram of the structure and localization of indicated tyrosines of FLT3 protein. (B) Beads with indicated immobilized peptides were incubated with cell lysates from Lnk-transfected COS-1 cells. After washing, the bound proteins were processed for Western blotting using anti-V5 Ab. (C) Immobilized phosphopeptides and their corresponding nonphosphorylated peptides were used to pull-down Lnk proteins and then were processed as above. (D-E) Immobilized phosphorylated and nonphosphorylated peptides, as above, were used to pull-down recombinant GST-fusion Lnk-domains, which were then processed for Western blotting using an anti-GST Ab. (F-G) Expression and phosphorylation of GST-fusion proteins of various domains of FLT3 in TKX1 E coli cells and the GST-fusion proteins were subsequently used to pull-down of cellular lysates from 293T cells overexpressing Lnk. GST indicates the GST control protein without fusion. DH5α indicates nonphosphorylated GST-fusion proteins expressed in DH5α E coli cells. (H) Pull-down of cellular lysates from 293T cells overexpressing either Lnk or the RE mutant with GST-FLT3-Kin2 proteins expressed and phosphorylated from TKX1 E coli cells. Input indicates that equal amounts of Lnk and RE mutant were used. (I) Pull-down of cellular lysates from 293T cells overexpressing Lnk with either wild-type or Y919F-mutated GST-FLT3-Kin2 proteins expressed and phosphorylated from TKX1 E coli cells. After pull-down, beads containing protein complex were processed for Western blotting using the indicated Abs: anti-phosphotyrosine (P-Ty), anti-GST, and anti-V5. Asterisk indicates nonspecific products. (J) COS-1 cells were cotransfected with Lnk and the FLT3-WT, FLT3-Y572/Y919F, or FLT3-Y591F/Y919F expression vectors. Cells were serum starved and stimulated with FL for 5 minutes. Lysates were immunoprecipitated with anti-FLT3 Abs and analyzed by Western blotting.
Tyr572, Tyr591, and Tyr919 residues of FLT3 were responsible for phosphorylation-dependent binding to Lnk. (A) Diagram of the structure and localization of indicated tyrosines of FLT3 protein. (B) Beads with indicated immobilized peptides were incubated with cell lysates from Lnk-transfected COS-1 cells. After washing, the bound proteins were processed for Western blotting using anti-V5 Ab. (C) Immobilized phosphopeptides and their corresponding nonphosphorylated peptides were used to pull-down Lnk proteins and then were processed as above. (D-E) Immobilized phosphorylated and nonphosphorylated peptides, as above, were used to pull-down recombinant GST-fusion Lnk-domains, which were then processed for Western blotting using an anti-GST Ab. (F-G) Expression and phosphorylation of GST-fusion proteins of various domains of FLT3 in TKX1 E coli cells and the GST-fusion proteins were subsequently used to pull-down of cellular lysates from 293T cells overexpressing Lnk. GST indicates the GST control protein without fusion. DH5α indicates nonphosphorylated GST-fusion proteins expressed in DH5α E coli cells. (H) Pull-down of cellular lysates from 293T cells overexpressing either Lnk or the RE mutant with GST-FLT3-Kin2 proteins expressed and phosphorylated from TKX1 E coli cells. Input indicates that equal amounts of Lnk and RE mutant were used. (I) Pull-down of cellular lysates from 293T cells overexpressing Lnk with either wild-type or Y919F-mutated GST-FLT3-Kin2 proteins expressed and phosphorylated from TKX1 E coli cells. After pull-down, beads containing protein complex were processed for Western blotting using the indicated Abs: anti-phosphotyrosine (P-Ty), anti-GST, and anti-V5. Asterisk indicates nonspecific products. (J) COS-1 cells were cotransfected with Lnk and the FLT3-WT, FLT3-Y572/Y919F, or FLT3-Y591F/Y919F expression vectors. Cells were serum starved and stimulated with FL for 5 minutes. Lysates were immunoprecipitated with anti-FLT3 Abs and analyzed by Western blotting.
As a parallel approach, GST-fusion proteins consisting of 5 consecutive domains of the intracellular part of FLT3 (Figure 3A) were expressed, purified, and phosphorylated in vitro by TKX1 E coli cells. In pull-down experiments using these 5 consecutive FLT3 domains incubated with Lnk purified from 293T cells, we found that Lnk exclusively bound to the JXM and Kinase-2 (Kin2) domain of FLT3 (Figure 3F), which contains Tyr572, Tyr591, and Tyr919, respectively. As expected, Lnk did not bind to the corresponding nonphosphorylated fusion proteins expressed from DH5α strains (Figure 3G). Because motif containing Tyr919 showed the most prominent binding activity, we generated Y919F point mutation of Kin2 domain (Kin2-Y919F) and found that Y919F mutation abolished binding of Lnk to the Kin2 domain (Figure 3I). Consistent with our co-IP results (Figure 2A and C), only Lnk, not the RE mutant, was able to interact with phosphorylated GST-FLT3-Kin2, reemphasizing the importance of the functional SH2 domain of Lnk (Figure 3H).
To verify further that FLT3 Tyr572, Tyr591, and Tyr919 were specific determinants for the interaction between Lnk and FLT3 in vivo, we generated 2 different FLT3 double mutants, Y572F/Y919F and Y591F/Y919F, and measured the interactions by co-IP. As shown in Figure 3J, interactions between Lnk and FLT3 double mutants were reduced substantially compared with FLT3-WT.
Lnk abolishes the activation of FLT3-WT and FLT3-ITD
Because Lnk has been reported to inhibit the activation of several tyrosine kinases, we next investigated whether Lnk regulates FLT3 activation by measuring the tyrosine phosphorylation of FLT3. 293T cells were cotransfected with FLT3-WT and either wild-type or mutant Lnk and immunoprecipitated with FLT3 Ab. We found that the tyrosine-phosphorylated form of FLT3-WT was remarkably reduced in the presence of Lnk compared with either the Lnk RE mutant or empty vector controls both before and after treatment with FL. The EQ mutant retained the ability to inhibit FLT3 activation, albeit to a lesser extent (Figure 4A). We further showed that Lnk inhibited FLT3-ITD phosphorylation (Figure 2D top panel) in a dose-dependent manner (Figure 4B).
Lnk attenuates phosphorylation of FLT3-WT/ITD. (A) 293T cells were cotransfected with various V5-tagged-Lnk constructs and FLT3. Cells were serum starved for 16 hours (−) and treated with FL for 15 minutes (+). (B) 293T cells were cotransfected with different amount of V5-tagged-Lnk and stable amount of FLT3-ITD and serum starved for 16 hours. Cellular lysates were prepared and precipitated with anti-FLT3 Ab, and both the supernatants and precipitates were subjected to Western blotting analysis using the indicated Abs.
Lnk attenuates phosphorylation of FLT3-WT/ITD. (A) 293T cells were cotransfected with various V5-tagged-Lnk constructs and FLT3. Cells were serum starved for 16 hours (−) and treated with FL for 15 minutes (+). (B) 293T cells were cotransfected with different amount of V5-tagged-Lnk and stable amount of FLT3-ITD and serum starved for 16 hours. Cellular lysates were prepared and precipitated with anti-FLT3 Ab, and both the supernatants and precipitates were subjected to Western blotting analysis using the indicated Abs.
Lnk negatively regulates downstream signaling of FLT3-WT/ITD
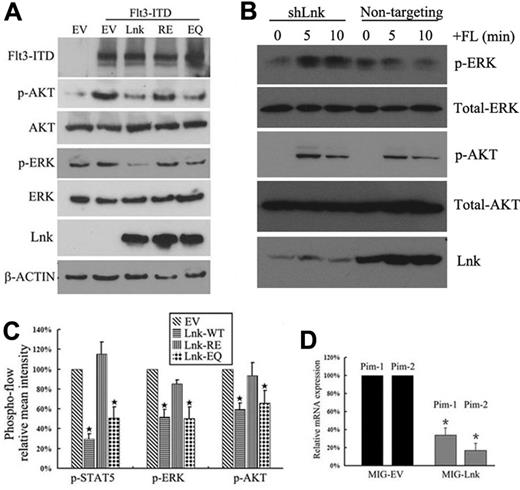
FLT3-ITD has been shown to aberrantly activate the MAPK, STAT5, and PI3K/AKT signaling pathways. We confirmed that forced expression of FLT3-ITD in 293T cells induced phosphorylation of AKT and ERK. Coexpression of the wild-type Lnk and EQ, but not the RE mutant, abolished the induction of p-AKT and p-ERK by FLT3-ITD (Figure 5A). We did not detect endogenous STAT5 expression in 293T cells.
Forced expression of Lnk inhibits FLT3-ITD–induced downstream signaling. (A) 293T cells were cotransfected with the indicated cDNAs for 32 hours and serum starved for 16 hours. Phosphorylation of ERK and AKT, as well as total ERK, AKT, and β-actin, were detected by Western blotting. (B) REH cells were transduced with lentiviral particles encoding either scramble shRNA (nontargeting) or shRNA against Lnk (shLnk) and selected for 2 weeks with puromycin. Stable cells were serum starved overnight and stimulated with FL for 5 or 10 minutes, and then subjected to Western blotting using the indicated Abs. (C) 32D/FLT3-ITD cells were transfected for 32 hours with various Lnk constructs coexpressing GFP (a sorting marker) and serum starved for 16 hours. After fixation and permeabilization, cells were analyzed for GFP expression and phosphorylation of STAT5, AKT, or ERK using flow cytometry. The mean intensities of each phosphorylated molecule are shown. (D) 32D/FLT3-ITD cells were transfected with either MIG empty vector (MIG-EV) or MIG-Lnk for 48 hours and serum starved for 16 hours. GFP+ cells were purified using flow cytometry and subjected to real-time PCR analysis for Pim1 and Pim2 mRNA expression. Data represent the means ± SD of 2 independent experiments.
Forced expression of Lnk inhibits FLT3-ITD–induced downstream signaling. (A) 293T cells were cotransfected with the indicated cDNAs for 32 hours and serum starved for 16 hours. Phosphorylation of ERK and AKT, as well as total ERK, AKT, and β-actin, were detected by Western blotting. (B) REH cells were transduced with lentiviral particles encoding either scramble shRNA (nontargeting) or shRNA against Lnk (shLnk) and selected for 2 weeks with puromycin. Stable cells were serum starved overnight and stimulated with FL for 5 or 10 minutes, and then subjected to Western blotting using the indicated Abs. (C) 32D/FLT3-ITD cells were transfected for 32 hours with various Lnk constructs coexpressing GFP (a sorting marker) and serum starved for 16 hours. After fixation and permeabilization, cells were analyzed for GFP expression and phosphorylation of STAT5, AKT, or ERK using flow cytometry. The mean intensities of each phosphorylated molecule are shown. (D) 32D/FLT3-ITD cells were transfected with either MIG empty vector (MIG-EV) or MIG-Lnk for 48 hours and serum starved for 16 hours. GFP+ cells were purified using flow cytometry and subjected to real-time PCR analysis for Pim1 and Pim2 mRNA expression. Data represent the means ± SD of 2 independent experiments.
To extend our findings to hematopoetic cells, a bicistronic retroviral vector containing green fluorescent protein (GFP) downstream of an IRES (MSCV-IRES-GFP, MIG) was used to introduce either wild-type or different mutants of Lnk into the 32D/FLT3-ITD cells. After transfection, cells were sorted for GFP and the activation of STAT5, ERK, and AKT was measured by flow cytometry with the relevant phosphospecific Abs (supplemental Figure 2). As shown in Figure 5C, expression of Lnk and the EQ mutant decreased the activities of all of these signal transducers, as reflected by their reduced phosphorylation levels. Pim-1/2 kinases are important downstream targets of FLT3-ITD and are involved in FLT3-ITD–mediated transformation.33–36 We found that the expression of these targets were down-regulated in Lnk-transfected 32D/FLT3-ITD cells (Figure 5D). Therefore, these data indicate that Lnk and the EQ mutant inhibit FLT3-ITD–mediated signaling pathways.
To investigate whether endogenous Lnk also regulates FLT3-WT–induced signaling events, we stably depleted Lnk in REH cells using lentiviral-based shRNA. As shown in Figure 5B, shRNA-targeting Lnk reproducibly resulted in enhanced FL-mediated ERK phosphorylation compared with nontargeting shRNA, which did not show appreciable p-ERK induction by FL. In contrast, FL-mediated activation of AKT remained unchanged in response to Lnk knockdown compared with control cells.
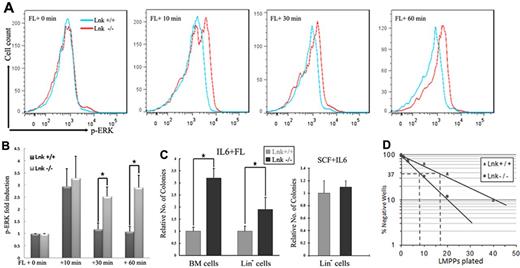
To verify further our findings in vivo, we measured the extent and duration of ERK and AKT activation after FL stimulation in purified FLT3+ BM cells from Lnk+/+ and Lnk−/− mice. FL induced stronger and more prolonged ERK activation in Lnk−/− FLT3+ BM cells than in Lnk+/+ FLT3+ BM cells (Figure 6A-B). In contrast, Lnk−/− FLT3+ BM cells exhibited induction of phosphorylated AKT comparable to Lnk+/+ FLT3+ BM cells after FL treatment (data not shown). These results suggest that Lnk acts as a physiologic negative regulator of FLT3-mediated MAPK-ERK signaling.
FL-dependent signaling and progenitor expansion are enhanced in Lnk−/− mice. (A-B) Primary BM cells from Lnk+/+ and Lnk−/− mice were cytokine starved for 6 hours and stimulated with FL for the indicated times. After fixation and permeabilization, cells were sorted by FLT3 expression. Phosphorylation of ERK was measured in FLT3+ cells using flow cytometry, and the mean intensity is shown. (C) Methylcellulose colony forming assays were performed with either primary BM cells or Lin− BM cells from Lnk+/+ and Lnk−/− mice in the presence of either FL + IL6 or SCF. Assays were performed in duplicate and data represent the means ± SD of 4 independent mice. (D) Limiting-dilution assay of sorted LMPPs (Flt3+Sca1+Kit+Lin−) was performed in the presence of FL and IL6. The frequencies of FL-responsive LMPPs (with 95% confidence limits) were 1 in 9 (7-11) and 1 in 18 (15-22) within the sorted cells isolated from Lnk−/− and Lnk+/+ mice, respectively.
FL-dependent signaling and progenitor expansion are enhanced in Lnk−/− mice. (A-B) Primary BM cells from Lnk+/+ and Lnk−/− mice were cytokine starved for 6 hours and stimulated with FL for the indicated times. After fixation and permeabilization, cells were sorted by FLT3 expression. Phosphorylation of ERK was measured in FLT3+ cells using flow cytometry, and the mean intensity is shown. (C) Methylcellulose colony forming assays were performed with either primary BM cells or Lin− BM cells from Lnk+/+ and Lnk−/− mice in the presence of either FL + IL6 or SCF. Assays were performed in duplicate and data represent the means ± SD of 4 independent mice. (D) Limiting-dilution assay of sorted LMPPs (Flt3+Sca1+Kit+Lin−) was performed in the presence of FL and IL6. The frequencies of FL-responsive LMPPs (with 95% confidence limits) were 1 in 9 (7-11) and 1 in 18 (15-22) within the sorted cells isolated from Lnk−/− and Lnk+/+ mice, respectively.
Loss of Lnk leads to enhanced levels of FL-responsive LMPPs
Blast colony-forming assays were performed on total BM cells to determine whether the loss of functional Lnk affected HPCs in vivo.37 In this assay, FL and IL6 are used to support the clonal growth of multipotent progenitors with myeloid and lymphoid potential, an activity restricted to cells positive for Kit, Sca1, and Flt3.37 Lnk−/− BM cells gave rise to more than 2 times more colonies than Lnk+/+ BM, suggesting a higher frequency of FL-responsive HPC in Lnk-deficient mice. Similar results were also obtained when BM cells were first enriched for lineage-negative (Lin−; Figure 6C) cells. To rule out the possibility that the increased cloning efficiency was because of altered sensitivity to IL6, we compared blast colony-forming capacity in the presence of SCF plus IL6; under these conditions, Lnk−/− HPCs gave rise to a similar number of colonies as Lnk+/+ progenitor cells (Figure 6C). We further showed that Lnk inhibited TPO- and FL-dependent, but not SCF-dependent, HPC expansion (supplemental Figure 3).
These results suggested that the LMPP38 compartment was expanded in Lnk-deficient mice. FACS analysis of Lnk−/− and Lnk+/+ confirmed a highly enriched Lin−Kit+Sca1+ population in Lnk-deficient mice, as reported previously,17,22,39 but only a minor increase in the size of the phenotypic LMPP population was observed (supplemental Figure 4A-B). However, limiting dilution assays of this population in the presence of FL and IL6 confirmed a nearly 2-fold increase in cloning efficiency of phenotypic LMPP in Lnk-deficient mice (Figure 6D). No increase in the size of the common lymphoid progenitor (IL7R+FLT3+KitmedSca1med) compartment nor in the proportion of B cell–primed cells within this compartment (Ly6dhi) was observed in Lnk-deficient mice (supplemental Figure 4C). In conclusion, Lnk is a critical regulator of FL-responsive multipotent progenitors that give rise to both myeloid and lymphoid progenitors, but its absence does not affect lymphoid progenitors that are marked by the expression of the IL7 receptor.
Expression of Lnk inhibits FLT3-WT/ITD/TKD–dependent proliferation of 32D cells
To analyze further the functional consequences of Lnk-mediated down-regulation of FLT-signaling, Lnk or various Lnk mutants were retrovirally transduced into 32D cells stably expressing FLT3-WT, FLT3-ITD, or FLT3-Y842C. FLT3-Y842C is one of the most common FLT3-TKD mutations found in AML patients. Similar to FLT3-ITD, FLT3-Y842C–transfected 32D cells showed constitutive FLT3 tyrosine phosphorylation and IL3-independent growth.40 Because GFP expression is correlated with the expression of Lnk, we were able to determine proliferation by analyzing the percentage of GFP+ cells in the cultures. In all 3 types of cells, the fraction of empty vector- and RE mutant–infected GFP+ cells did not change throughout the experiment. In contrast, a dramatic decrease in the percentage of GFP+ cells was observed in Lnk- and EQ mutant–infected cells (Figure 7). We further examined the proliferation of 32D/FLT3-ITD cells in the presence of IL3 and found that IL3 reverted the growth retardation caused by Lnk and the EQ mutant (Figure 7C), indicating that Lnk and the EQ mutant inhibited FLT3-dependent cell growth specifically through down-regulating FLT3-induced signaling.
Lnk inhibits FLT3-WT/ITD/TKD–driven proliferation of 32D cells. 32D cells stably expressing FLT3-WT (A), FLT3-TKD (B), or FLT3-ITD (C) were infected with either retroviral empty vector (EV), wild-type Lnk (WT), R364E mutant (RE), or E208Q mutant (EQ) coexpressing GFP. GFP+ cells were measured daily by flow cytometry and calculated as percentage of GFP+ cells present at 48 hours after infection. In 32D/FLT3-ITD cells, assays were also performed in the presence of IL3. Data represent the means ± SD of 3 independent experiments.
Lnk inhibits FLT3-WT/ITD/TKD–driven proliferation of 32D cells. 32D cells stably expressing FLT3-WT (A), FLT3-TKD (B), or FLT3-ITD (C) were infected with either retroviral empty vector (EV), wild-type Lnk (WT), R364E mutant (RE), or E208Q mutant (EQ) coexpressing GFP. GFP+ cells were measured daily by flow cytometry and calculated as percentage of GFP+ cells present at 48 hours after infection. In 32D/FLT3-ITD cells, assays were also performed in the presence of IL3. Data represent the means ± SD of 3 independent experiments.
Discussion
Lnk controls FLT3-WT function
In the present study, we identified both FLT3-WT and FLT3-ITD as novel binding partners of the adaptor Lnk. We demonstrated that overexpression of Lnk in 293T cells attenuated FLT3 tyrosine phosphorylation induced by FL. We also showed that MAPK-ERK signaling stimulated by FL was amplified in primary BM cells from Lnk-knockout mice. Lnk deficiency led to enhanced FL-induced LMPP cell expansion and proliferation. Furthermore, we found that expression of Lnk inhibited FLT3-dependent 32D cell proliferation.
Through affinity fishing, GST pull-down, and co-IP assays of FLT3 double mutants with Lnk, we identified Tyr572, Tyr591, and Tyr919 as specific determinants for this interaction. The fact that purified GST-SH2-Lnk, but not GST-PH-Lnk, was able to bind to these phosphopeptides in vitro indicated the direct binding between Lnk and FLT3. We reported previously that Lnk binds to the JXM domain of c-Kit and specifically to p-Tyr568. It is worth noting that Tyr591 of FLT3 and Tyr568 of c-Kit are conserved tyrosines in the JXM domain, the role of which is well defined (supplemental Figure 1). Conversely, the interaction of Lnk with p-Tyr919 is worth investigating further for the following reasons: (1) the binding activity to Lnk is much stronger than the other 2 tyrosines; (2) the conserved residue in murine Flt3 (p-Tyr922) has been shown to be critical for constitutive activation of murine FLT3 mutant41 ; and (3) the corresponding residues in both c-Kit (p-Tyr900) and the PDGFR-β (p-Tyr934) have been shown to play important roles in maintaining kinase activity.43,44
FLT3 is expressed in almost all human hematopoietic stem cells and HPCs that give rise to lymphoid or myeloid lineages or both.44 In contrast, in the mouse, high Flt3 expression is restricted to LMPP and lymphoid-restricted progenitors, although reduced levels are found in a small fraction of early granulocyte/monocyte progenitors.45,46 In concordance with this expression pattern and in support of Lnk-mediated restriction of FLT3-signaling, we observed an approximately 2-fold increase in FL-stimulated clonogenic HPCs, corresponding to a LMPP phenotype, in Lnk-deficient mice. We could not detect an increase in the common lymphoid progenitor compartment, although the importance of FL stimulation in maintaining this compartment is well established.45 Conceivably, additional controls may limit the expansion of this population in vivo. Nevertheless, our data demonstrate that Lnk is a physiologic negative regulator of FLT3-stimulated HPCs, a role that may be even more pronounced in human hematopoiesis.
Lnk inhibits FLT3-ITD–induced aberrant signaling
Mutation in the FLT3 gene is one of the most frequent events in AML, and understanding the consequences of these mutations on cell proliferation and survival signals is paramount to developing therapeutic reagents. Several studies have demonstrated differential signaling outcomes of AML-associated FLT3-ITD compared with FLT3-WT, which are also reflected in quantitative and qualitative differences in the phosphorylation pattern of FLT3-WT and AML-associated FLT3-ITD.47,48 Nevertheless, our present data suggest that the signaling events induced by FLT3-WT and FLT3-ITD are both under the control of Lnk. The major signal transduction pathways activated by FLT3-ITD include PI3K/AKT, RAS/MAPK, and STAT5. By measuring AKT, ERK, and STAT5 phosphorylation via phosphospecific flow cytometry, we conclude that all 3 signaling pathways were constrained by Lnk. Consistent with this, overexpression of Lnk suppressed the FLT3-ITD–dependent proliferation of 32D cells. The fact that Lnk also inhibits FLT3-TKD–induced myeloid cell growth indicates that Lnk is an important negative regulator of different FLT3 variants.
The Lnk E208Q mutation has been found in MPN patients and has been suggested to retain near-complete inhibitory capacity against JAK2-induced signaling.28 Because FLT3 mutations are also implicated in MPN pathogenesis, we investigated herein whether the EQ mutant binds and regulates FLT3-ITD activation. Our results indicate that this mutant interacts with both FLT3-WT and FLT3-ITD. Overexpression of the EQ mutant attenuates FLT3-ITD–induced signaling pathways, although to a lesser extent compared with wild-type Lnk. Similarly, partial inhibition was noticed in the 32D growth assays, suggesting that the EQ mutant exhibits a subtle loss of function.
FLT3 mutations are important molecular targets for the development of therapeutics that could be used to treat AML and other forms of leukemia. However, when used as monotherapy in clinical trials, FLT3 inhibitors have shown limited responses. Recently, intracellular delivery of the Lnk protein was accomplished through octa-arginine modification of the protein, allowing efficient inhibition of TPO-induced leukemic cell growth by promoting apoptosis.49 Therefore, the results of the present study may provide a novel therapeutic approach for developing treatments for FLT3-mutation–asscociated hematologic malignancies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank C. H. Brandts for providing FLT3-related constructs and cell lines.
This work was supported by the National Institutes of Health (grants R01CA026038-32, U54CA143930, and 2P01 HL073104-060); the Agency for Science, Technology, and Research (A*STAR; investigator grant to H.P.K.); and by the Oncogenic Networks funded by the Deutsche Krebshilfe Stiftung (to C.S.). The Heinrich-Pette-Institute belongs to the Leibniz Association and is financed by the Freie und Hansestadt Hamburg and the German Federal Ministry of Health.
National Institutes of Health
Authorship
Contribution: D.-C.L. designed the research, performed the experiments, analyzed the results, and wrote the manuscript; T.Y., M.K.-M., L.-W.D., S. Gueller, S. Gery, and T.T. analyzed the results; U.B. performed the colony assays and assisted in the FACS analysis; J.U.K. and L.R. performed the affinity fishing; and C.S. and H.P.K. designed the research, analyzed the results, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: De-Chen Lin, PhD, Cancer Science Institute of Singapore, 14 Medical Drive, 117599, Singapore; e-mail: dchlin10@gmail.com.
References
Author notes
C.S. and H.P.K. contributed equally to this work.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal