Abstract
CD4+ T cells play a central role in adaptive immunity. The acknowledgment of their cytolytic effector function and the finding that endogenous antigens can enter the HLA class II processing pathway make CD4+ T cells promising tools for immunotherapy. Expression of HLA class II and endogenous antigen, however, does not always correlate with T-cell recognition. We therefore investigated processing and presentation of endogenous HLA class II epitopes that induced CD4+ T cells during in vivo immune responses. We demonstrate that the peptide editor HLA-DM allowed antigen presentation of some (DM-resistant antigens) but abolished surface expression of other natural HLA class II epitopes (DM-sensitive antigens). DM sensitivity was shown to be epitope specific, mediated via interaction between HLA-DM and the HLA-DR restriction molecule, and reversible by HLA-DO. Because of the restricted expression of HLA-DO, presentation of DM-sensitive antigens was limited to professional antigen-presenting cells, whereas DM-resistant epitopes were expressed on all HLA class II–expressing cells. In conclusion, our data provide novel insights into the presentation of endogenous HLA class II epitopes and identify intracellular antigen processing and presentation as a critical factor for CD4+ T-cell recognition. This opens perspectives to exploit selective processing capacities as a new approach for targeted immunotherapy.
Introduction
CD4+ T cells are important in antitumor immunity as helper cells for the induction and maintenance of cytotoxic CD8+ T cells.1–3 Moreover, evidence is emerging that CD4+ T cells alone can provide efficient antitumor reactivity.4–7 In a NOD/SCID mouse model for human acute lymphoblastic leukemia, infusion of human CD4+ T cells led to eradication of the malignant cells.8 Clinical trials in allogeneic stem cell transplantation (alloSCT) found that depletion of CD8+ T cells from donor lymphocyte infusion preserved the ability of the T cells to induce conversion to donor chimerism,9,10 highlighting the direct effector function of CD4+ T cells.
Antigen presentation to T cells by HLA surface molecules depends on protein degradation mediated by proteasomes and lysosomal proteases as the 2 major cellular breakdown mechanisms. Traditionally, it has been assumed that HLA class II molecules present exogenous antigens which are degraded by proteases within the endosomal/lysosomal system, whereas endogenous cytosolic antigens are degraded by the proteasome and presented by HLA class I molecules. In the past decade, however, evidence emerged that HLA class I molecules can also present exogenous antigens via a process called cross-presentation,11 and that intracellular antigens can enter the HLA class II pathway.12,13
In antitumor immunity, professional APCs such as dendritic cells (DCs) take up and present antigens derived from tumor cells. The encounter of these exogenous antigens presented on DCs may be sufficient for the induction of CD4+ T cells and their function as helper cells. However, to serve as specific targets for cytolytic CD4+ T cells, tumor cells need to present endogenous peptides in HLA class II molecules as expressed at their cell surface. We and others, however, observed that some HLA class II–positive target cells are not recognized by the respective CD4+ T-cell clones,14,15 despite appropriate HLA class II and antigen expression. Already in 1996, Harris et al provided evidence for cell type–specific differences in presentation of endogenous antigens in HLA class II.16 By eluting peptides from HLA-DR, professional APCs were shown to present an extended repertoire of self-peptides17,18 compared with nonprofessional APCs. The investigators also found that significant amounts of class II–associated invariant chain peptide (CLIP), the remnant of the invariant chain (Ii), were presented at the cell surface of professional APCs, whereas CLIP was absent on nonprofessional APCs.16 Surface expression of CLIP on professional APCs may be attributed to a molecule called HLA-DO. Although its precise biologic role is still unclear, HLA-DO has been shown to increase CLIP surface presentation by inhibition of HLA-DM in a pH-dependent manner,19,20 and its expression has been reported to be confined to professional APCs, such as B cells, DCs, and thymic epithelial cells.21
Here, we investigated whether endogenous antigens identified as natural targets for CD4+ T cells are differentially processed and presented on HLA class II–positive cells. We previously identified 6 antigens derived from endogenous proteins as targets for CD4+ T cells during in vivo immune responses after alloSCT. These antigens comprise 5 HLA class II–restricted autosomal minor histocompatibility antigens (MiHAs)14,22 and 1 male-specific MiHA.23 We analyzed the contribution of the Ii, HLA-DM, and HLA-DO to the processing and presentation of these antigens via HLA class II, and we have shown that natural HLA class II epitopes can be divided into 2 groups. For 1 group of antigens, presentation is preserved in the presence of HLA-DM (DM-resistant), whereas for another set of antigens presentation is abolished by expression of HLA-DM (DM-sensitive). Presentation of DM-sensitive antigens in HLA class II molecules can be restored by coexpression of HLA-DO, suggesting that in vivo presentation of DM-sensitive antigens is restricted to professional APCs. In conclusion, our study suggests the existence of a category of endogenous HLA class II epitopes in vivo that behave like CLIP and whose presentation depends on a delicate balance between HLA-DM and DO. This insight opens possibilities for the use of these antigens for selective targeting of specific types of HLA class II–positive cells in (antitumor) immune responses.
Methods
Hematopoietic cell isolation
PBMC and bone marrow samples were obtained from healthy persons after approval by the Leiden University Medical Center Institutional Review Board and informed consent according to the Declaration of Helsinki. Mononuclear cells were isolated by Ficoll-Isopaque separation and cryopreserved. CD14+ monocytes were isolated from PBMCs by magnetic beads according to the manufacturer's instructions (Miltenyi Biotec).
Cell culture
HeLa, MJS, Raji, COS, and EBV-transformed B-cell lines (EBV-LCLs) were cultured in IMDM (Lonza BioWhittaker) with 10% FCS (Cambrex), 1% penicillin/streptomycin (Lonza), and 1.5% l-glutamine (Lonza). T-cell clones were cultured in IMDM with 5% human serum, 5% FCS, and 100 IU/mL IL-2 (Chiron) and were restimulated every 10-20 days with irradiated allogeneic PBMCs and 0.8 μg/mL PHA (Oxoid).22
Immature DCs were generated by culturing monocytes in medium with 100 ng/mL GM-CSF (Novartis) and 500 IU/mL IL-4 (Schering-Plough) for 7 days. During the final 2 days 100 ng/mL GM-CSF, 10 ng/mL TNF-α (Cellgenix), 10 ng/mL IL-1β (Cellgenix), 10 ng/mL IL-6 (Cellgenix), 1 μg/mL prostaglandin E2 (Sigma-Aldrich), and 500 IU/mL IFN-γ (Boehringer-Ingelheim) were added for maturation.
Fibroblasts were cultured in DMEM with low glucose (Lonza), 8% FCS, 1% penicillin/streptomycin, and 1.5% glutamine with or without IFN-γ (100 IU/mL) for 4 days.
T-cell library
CD4+/CD56− T cells were isolated by flow cytometry (Aria; BD Biosciences) and seeded at 2000 cells/well and expanded with 500 IU/mL IL-2, 0.8 μg/mL PHA, and 50 000 irradiated allogeneic feeders per well.24
After 2-3 weeks of expansion CD4+ T-cell bulks were washed twice and plated at 2 × 105 cells/well for each target. As target cells HeLa-Ii and HeLa-Ii/DM cotransduced with the relevant HLA class II molecules were used.
Flow cytometry
Flow cytometric analyses were performed on a FACSCalibur (BD Biosciences) and cell sorting on a FACSAria (BD Biosciences) with the use of PE-labeled monoclonal antibodies against HLA-DR (L243; BD Biosciences), CLIP (Cer.CLIP; Santa Cruz Biotechnology), NGFR (C40-1457; BD PharMingen), CD80 (L307.4; BD Biosciences), HLA-DQ (1a3; Meridian Life Science), CD20 (L27; BD Biosciences), and CD4 (RPA-T4; BD PharMingen). FITC-labeled anti-CD14 (M5E2) and anti-CD34, (581) APC-labeled anti-CD19 (HIB19) and anti-CD56 (B159), and peridinin chlorophyll protein complex–labeled anti-CD3 (SP34-2) were purchased from BD PharMingen.
Retroviral constructs and transduction
Ii, HLA-DMα, and HLA-DMβ were cloned into pLZRS vectors. For Ii, CD80 was used as marker gene, for HLA-DMα truncated ΔNGFR, and for HLA-DMβ enhanced green fluorescence protein. As control pLZRS with only CD80 was cloned, and the Ii was recloned in MP71 with CD20 as marker gene. HLA-DQB1*0603, DQB1*0501, DQA*0103, and DQA*0101 were cloned into pLZRS vectors with enhanced green fluorescence protein (α chains) and truncated ΔNGFR (β chains). HLA-DRB1*0301, DRB1*1301, DRB3*0101, DRB3*0202, and DRA*0102 were cloned in MP71 vectors with the use of truncated ΔNGFR as marker gene.
Full-length PTK2B, MR1, PI4K2B, DBY, and MTHFD1 were cloned into MP71 with CD20 as marker gene. HLA-DOα was cloned into MP71 with bicistronic HLA-DQA*0103 and HLA-DOβ with bicistronic HLA-DQB1*0603. Chimeric constructs and mutated DRα chains were cloned by 2-step PCR. For chimera A the 39-bp sequence of the PTK2B epitope was inserted at positions 220-262 bp in the PI4K2B sequence (NM_018323), whereas the 42-bp sequence of the PI4K2B epitope was inserted at positions 2371-2410 bp in the PTK2B sequence (NM_173175) for chimera B. Mutated DRα chains were cloned by inserting single-point mutations at position 193 (G/A) and 227 (T/C), respectively.25 Chimeric constructs were cloned into MP71 with CD20, mutated DRA in MP71 vectors with ΔNGFR. All constructs were verified by sequencing. Retroviral supernatant fluid was obtained by transfecting wild-type Φnx-A packaging cells as previously described,26 with the exception that the Fugene HD transfection kit (Roche) was used. Viral supernatant fluids were used for transduction on plates coated with recombinant human fibronectin CH 296 (Takara Shuzo).26
Antigen presentation assays
Stimulator cells (3 × 104 cells/well) were coincubated with CD4+ T-cell clones (5 × 103 cells/well) overnight at 37°C in 96-well plates. For recombinant protein loading Escherichia coli cells with a pKE-1 vector encoding the respective antigen under an isopropyl thiogalactoside–inducible promoter were grown to OD600 of 0.5 with 50 μg/mL ampicillin (Sigma-Aldrich), and protein expression was induced by 1mM isopropyl thiogalactoside for 4 hours (Promega). Subsequently, bacteria were opsonized for 1 hour by adding human serum with 17% (v/v) complement (Sigma-Aldrich). Target cells (3 × 104 cells/well) were pulsed with complement-opsonized bacteria in culture medium with 30 μg/mL gentamycin (Sigma-Aldrich) overnight at 37°C. T-cell clones were added, and cytokine release was measured after overnight incubation in 25-μL supernatant fluids by IFN-γ ELISA (Sanguin).
Microarray gene expression analysis
Total RNA was isolated with small and micro scale RNAqueous isolation kits (Ambion) and was amplified with the TotalPrep RNA amplification kit (Ambion). After preparation with the use of the whole-genome gene expression direct hybridization assay (Illumina), cRNA samples were dispensed onto Human HT-12 Version 3 Expression BeadChips (Illumina). Hybridization was performed in the Illumina hybridization oven for 17 hours at 58°C. Microarray gene expression data were analyzed with R 2.15 (R Project). Normalization was done in the lumi package,27 using the variance stabilizing transformation and quantile normalization. All microarray data are available at the Gene Expression Omnibus under accession no. GSE38798.
Results
HLA-DM has divergent effects on the presentation of natural HLA class II epitopes.
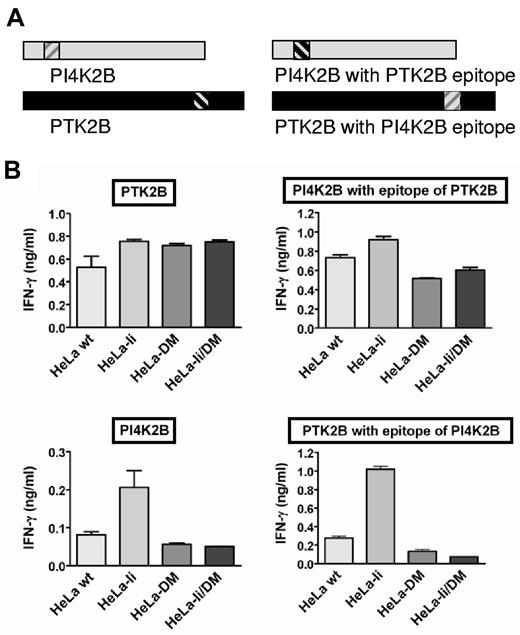
To study processing and presentation of endogenous HLA class II epitopes, we selected 6 polymorphic antigens identified as targets for CD4+ T cells during in vivo–induced immune responses after HLA-matched alloSCT. Because of single amino acid differences between patient and donor, endogenous peptides of the patient presented in shared HLA molecules are recognized by donor T cells. Five of these polymorphic peptides were derived from autosomal proteins (LY75, MR1, PTK2B, PI4K2B, and MTHFD1) and were identified as targets for MiHA-specific CD4+ T cells.14,22 One male-specific MiHA was derived from the DBY protein.23 The 6 proteins included 2 transmembrane proteins (LY75 and MR1), 2 cytosolic kinases (PTK2B and PI4K2B), 1 cytosolic enzyme (MTHFD1), and 1 nuclear/cytosolic RNA helicase (DBY). The antigens were restricted by 4 different HLA-DR and 2 different HLA-DQ molecules: DRB1*0301/A*0102 (MTHFD1), DRB1*1301/A*0102 (LY75), DRB3*0101/A*0102 (PTK2B), DRB3*0202/A*0102 (MR1), DQB1*0603/A*0103 (PI4K2B), and DQB1*0501/A*0101 (DBY). To investigate the role of molecules with known functions in processing and presentation of HLA class II epitopes, we retrovirally transduced the HLA class II–negative cervix carcinoma HeLa cell line with the genes encoding the Ii, HLA-DMα/β, or both. Cell lines were sorted by flow cytometry on the basis of marker gene expression for Ii (CD80) and HLA-DMα/β (GFP/NGFR). Subsequently, all HeLa variants were transduced with the appropriate HLA class II and isolated on the basis of HLA-DR or -DQ surface expression. Expression of the Ii and HLA-DM in the transduced cell lines was confirmed by Western blot analysis (data not shown), and their functionality was confirmed by monitoring cell surface expression of CLIP, the remnant of the Ii occupying the peptide binding groove. Whereas surface expression of the introduced DR or DQ restriction alleles was comparable between the different HeLa variants (Figure 1A; data not shown), staining of CLIP in DR was markedly increased on HeLa cells stably expressing the Ii and completely removed by coexpression of HLA-DM (Figure 1A).
Natural HLA class II epitopes can be divided in HLA-DM–sensitive and –resistant antigens. The cervix carcinoma HeLa cell line was retrovirally transduced with the invariant chain (Ii), HLA-DM, or a combination of both. (A) Indicated is the HLA-DR (left) and class II–associated invariant chain peptide (CLIP; right) surface expression as measured by flow cytometry. (B) All HeLa variants were cotransduced with the appropriate HLA class II restriction molecules, and specific release of IFN-γ by the CD4+ T-cell clones after exogenous loading with recombinant proteins was measured in ELISA. Antigens in the upper row display a DM-resistant phenotype as defined by retained antigen presentation on expression of HLA-DM, whereas DM-sensitive antigens with impaired presentation in the presence of HLA-DM (DM-sensitive) are depicted in the lower part. Mean ± SD release of IFN-γ in duplicate wells is shown. (C) HLA-DM was retrovirally transduced into EBV-transformed B-cell lines (EBV-LCL) from patient MRJ, and tested for T-cell recognition in IFN-γ ELISA. CD4+ T-cell clones specific for PI4K2B, MR1, PTK2B, LY75, and MTHFD1 were isolated from patient MRJ and selected on the basis of recognition of patient-derived EBV-LCL. HLA-DM was also introduced into the HLA-DQB1*0502–positive Raji cell line derived from a male patient with Burkitt lymphoma to measure T-cell recognition of endogenous DBY antigen. Solid squares and open circles indicate T-cell recognition of the endogenous antigens as expressed in the wild-type and HLA-DM transduced EBV-LCL, respectively. Numbers of titrated target cells are depicted on the x-axis. Mean ± SD release of IFN-γ in duplicate wells is shown.
Natural HLA class II epitopes can be divided in HLA-DM–sensitive and –resistant antigens. The cervix carcinoma HeLa cell line was retrovirally transduced with the invariant chain (Ii), HLA-DM, or a combination of both. (A) Indicated is the HLA-DR (left) and class II–associated invariant chain peptide (CLIP; right) surface expression as measured by flow cytometry. (B) All HeLa variants were cotransduced with the appropriate HLA class II restriction molecules, and specific release of IFN-γ by the CD4+ T-cell clones after exogenous loading with recombinant proteins was measured in ELISA. Antigens in the upper row display a DM-resistant phenotype as defined by retained antigen presentation on expression of HLA-DM, whereas DM-sensitive antigens with impaired presentation in the presence of HLA-DM (DM-sensitive) are depicted in the lower part. Mean ± SD release of IFN-γ in duplicate wells is shown. (C) HLA-DM was retrovirally transduced into EBV-transformed B-cell lines (EBV-LCL) from patient MRJ, and tested for T-cell recognition in IFN-γ ELISA. CD4+ T-cell clones specific for PI4K2B, MR1, PTK2B, LY75, and MTHFD1 were isolated from patient MRJ and selected on the basis of recognition of patient-derived EBV-LCL. HLA-DM was also introduced into the HLA-DQB1*0502–positive Raji cell line derived from a male patient with Burkitt lymphoma to measure T-cell recognition of endogenous DBY antigen. Solid squares and open circles indicate T-cell recognition of the endogenous antigens as expressed in the wild-type and HLA-DM transduced EBV-LCL, respectively. Numbers of titrated target cells are depicted on the x-axis. Mean ± SD release of IFN-γ in duplicate wells is shown.
The 4 HeLa variants cotransduced with the relevant HLA class II restriction molecules were pulsed with recombinant proteins to induce antigen expression. Protein uptake, processing, and subsequent surface presentation of the peptide were quantified by IFN-γ release of the specific CD4+ T-cell clones. Presentation of all antigens was increased by expression of the Ii. Coexpression of HLA-DM did not affect or slightly increased presentation of 3 antigens (MTHFD1, PTK2B, and DBY), whereas recognition of the other 3 antigens (LY75, MR1, and PI4K2B) was abolished in the presence of HLA-DM (Figure 1B). To exclude that the observed increase in recognition of the HeLa-Ii variant was because of expression of the marker gene CD80, we recloned the Ii in a construct with CD20 and yielded the same results (data not shown).
To confirm the divergent effect of HLA-DM on presentation of endogenously expressed antigens, we retrovirally transduced HLA-DM into patient-derived EBV-LCL. As described earlier these EBV-LCL expressed all HLA class II epitopes from LY75, MR1, PTK2B, PI4K2B, and MTHFD1 and were strongly recognized by the specific T-cell clones. In addition, to study presentation of the male antigen DBY, HLA-DM was transduced into the Raji cell line, which is derived from a male patient with Burkitt lymphoma and expresses HLA-DQB1*05 and DBY. Similar as observed for the HeLa variants pulsed with recombinant proteins, T-cell recognition of the endogenously expressed antigens could be again divided in 2 groups. Recognition of MTHFD1, PTK2B, and DBY was not affected by overexpression of HLA-DM, whereas presentation of LY75, MR1, and PI4K2B was markedly decreased in the presence of transduced HLA-DM (Figure 1C). Finally, we confirmed the effect of HLA-DM in another cell line. Antigen-negative COS cells were transduced with Ii alone or Ii in combination with HLA-DM and subsequently with the HLA-DR restriction allele for PTK2B or the HLA-DQ restriction allele for PI4K2B. Next, the genes encoding PTK2B or PI4K2B were introduced, and T-cell recognition was measured by IFN-γ ELISA. The data confirmed that HLA-DM did not affect T-cell recognition of DM-resistant antigen PTK2B, whereas recognition of DM-sensitive antigen PI4K2B was significantly inhibited (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
In conclusion, these data indicated the existence of 2 different groups of natural HLA class II epitopes. Presentation of 1 group of antigens is not or hardly influenced by HLA-DM (DM-resistant), whereas the other group of natural HLA class II epitopes behaves like CLIP and their presentation is removed by HLA-DM (DM-sensitive). This difference in behavior toward HLA-DM cannot be explained by the binding affinity of the peptides for their HLA class II restriction molecules or affinity of the T-cell receptors as expressed on the different T-cell clones, because no consistent difference in these variables between DM-resistant and DM-sensitive antigens was observed (supplemental Figure 2). Moreover, because the effect of HLA-DM on T-cell recognition of HLA class II–restricted antigens was measured after retroviral transfer into the same target cells, variables such as density of HLA class II surface molecules and avidity of the T-cell clones as influenced by adhesion and costimulatory molecules can be excluded as relevant factors.
HLA-DM sensitivity was epitope specific
To analyze whether the sensitivity or resistance toward HLA-DM was a characteristic of the protein or the epitope, we made wild-type and epitope exchange constructs of PTK2B and PI4K2B (Figure 2A). The T-cell epitope of DM-resistant antigen PTK2B (a 13-aa epitope in HLA-DRB3*0101) was exchanged with the T-cell epitope of DM-sensitive antigen PI4K2B (a 14-aa epitope in HLA-DQB1*0603) and vice versa. The constructs were transduced in HeLa cells expressing HLA-DRB3*0101/A*0102 and DQB1*0603/A*0103, respectively. T-cell recognition assays found that exchange of the T-cell epitopes still allowed presentation in the context of their original HLA class II restriction molecule. Moreover, the exchanged T-cell epitopes showed the same behavior toward HLA-DM as in their natural configuration (Figure 2B). Thus, DM sensitivity is an intrinsic property of a T-cell epitope that does not depend on the context of the epitope within the protein.
DM sensitivity is determined by the T-cell epitope. (A) Schematic overview of wild-type proteins PI4K2B and PTK2B (left) and proteins with exchanged T-cell epitopes (right). T-cell epitopes are indicated by the hatched areas. (B) T-cell recognition in IFN-γ ELISA is shown for the 2 wild-type PI4K2B and PTK2B antigens (left) and proteins with exchanged T-cell epitopes (right) after retroviral transduction into HeLa variants cotransduced with DRB3*0101/DRA*0102 (top) and DQB1*0603/DQA*0103 (bottom), respectively. Mean ± SD release of IFN-γ of duplicate wells on coincubation with the specific CD4+ T-cell clones is shown.
DM sensitivity is determined by the T-cell epitope. (A) Schematic overview of wild-type proteins PI4K2B and PTK2B (left) and proteins with exchanged T-cell epitopes (right). T-cell epitopes are indicated by the hatched areas. (B) T-cell recognition in IFN-γ ELISA is shown for the 2 wild-type PI4K2B and PTK2B antigens (left) and proteins with exchanged T-cell epitopes (right) after retroviral transduction into HeLa variants cotransduced with DRB3*0101/DRA*0102 (top) and DQB1*0603/DQA*0103 (bottom), respectively. Mean ± SD release of IFN-γ of duplicate wells on coincubation with the specific CD4+ T-cell clones is shown.
HLA-DM precluded presentation of DM-sensitive epitopes via interaction with the HLA-DR α chain
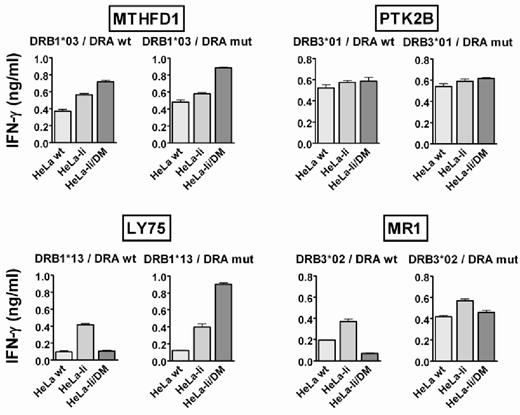
Removal of CLIP by HLA-DM has been described to be mediated via interaction between HLA-DM and HLA-DR, and certain amino acid residues in the HLA-DRα and β chain have been described as indispensable for HLA-DM to elicit its function.25 We investigated whether the same interaction between HLA-DR and HLA-DM was required for removal of the natural DM-sensitive HLA class II epitopes. Because all HLA-DR–restricted antigens use the same α chain (DRA*0102), we mutated a known interaction residue within this molecule (E40K). This mutation hampered the formation of SDS-stable DR dimers (data not shown), indicating loss of interaction with HLA-DM.25 For T-cell recognition, HeLa variants transduced with wild-type or mutated DRα were pulsed with recombinant proteins to induce antigen expression. No effect on presentation of DM-resistant antigens MTHFD1 and PTK2B was observed. However, the mutation rescued presentation of the DM-sensitive antigens LY75 and MR1 in the presence of HLA-DM (Figure 3). Mutation of another interaction residue within DRα (F51S) gave similar results (data not shown). Peptide loading showed that the mutation neither interfered with recognition of the HLA-peptide complex by the T-cell receptor nor prohibited binding of the peptide to the HLA molecule (data not shown). In conclusion, our data indicated that DM sensitivity of natural T-cell epitopes is mediated via interaction between HLA-DM and the HLA class II restriction molecule in a manner similar as described for CLIP.
HLA-DM sensitivity is mediated via interaction with HLA-DRα. The effect of point mutation E40K in DRα (DRA mut) versus wild-type DRα (DRA wt) was tested on the presentation of DM-resistant antigens MTHFD1 and PTK2B (top) as well as DM-sensitive antigens LY75 and MR1 (bottom). HeLa variants were transduced with the genes for DRA wt or DRA mut in combination with the appropriate DRB chains and tested for T-cell recognition after exogenous loading of the indicated recombinant proteins. Mean ± SD release of IFN-γ after coincubation with the specific T-cell clones is depicted.
HLA-DM sensitivity is mediated via interaction with HLA-DRα. The effect of point mutation E40K in DRα (DRA mut) versus wild-type DRα (DRA wt) was tested on the presentation of DM-resistant antigens MTHFD1 and PTK2B (top) as well as DM-sensitive antigens LY75 and MR1 (bottom). HeLa variants were transduced with the genes for DRA wt or DRA mut in combination with the appropriate DRB chains and tested for T-cell recognition after exogenous loading of the indicated recombinant proteins. Mean ± SD release of IFN-γ after coincubation with the specific T-cell clones is depicted.
HLA-DO restored presentation of DM-sensitive antigens
HLA-DO has been described as a natural inhibitor of HLA-DM, and its expression has been mainly confined to B cells, mature DCs, and thymic epithelial cells. We therefore investigated whether expression of HLA-DO could restore presentation of DM-sensitive antigens in HLA-DM–positive cells. We transduced the HLA-DOα/β chains into the HLA-DO–negative and HLA class II–positive melanoma MJS cell line, which endogenously expresses Ii and HLA-DM. The functionality of the HLA-DO construct was confirmed by occurrence of CLIP surface expression (Figure 4A). MJS cells endogenously express HLA-DRB3*0101, and we retrovirally introduced HLADQB1*0603/A*0103 either alone or in combination with HLA-DO. MJS cells with and without HLA-DO were subsequently loaded with recombinant PTK2B and PI4K2B proteins or retrovirally transduced with the genes encoding these proteins and were compared for presentation of the HLA class II epitopes. HLA-DO increased presentation of the DM-sensitive antigen PI4K2B, whereas it did not alter presentation of DM-resistant antigen PTK2B (Figure 4B-C).
HLA-DM sensitivity is reversible by HLA-DO. (A) Histograms are shown for class II–associated invariant chain peptide (CLIP) surface expression on MJS wt (open) and MJS cells transduced with HLA-DOα/β (filled) as measured by flow cytometry. (B) The influence of HLA-DO on presentation of the DM-sensitive antigen PI4K2B and DM-resistant antigen PTK2B was analyzed by testing T-cell recognition of MJS wt (open bars) and MJS transduced with HLA-DOα/β (closed bars). MJS melanoma cells endogenously express the invariant chain and HLA-DM but lack expression of HLA-DO. The HLA-DRB3*0101 restriction allele for PTK2B is endogenously expressed by MJS cells and the HLA-DQB1*0603/DQA1*0103 restriction allele for the HLA class II epitope from PI4K2B was introduced retrovirally. To induce antigen expression, MJS cells were loaded with recombinant proteins in 2 different concentrations. Mean ± SD release of IFN-γ in duplicate wells is shown. (C) MJS-DQ6 (open bars) and MJS-DQ6-DO (closed bars) cells were transduced with retroviral vectors encoding PI4K2B and PTK2B to test the influence of HLA-DO on endogenously expressed antigens. T-cell recognition was tested against titrated numbers of target cells (10 000 and 3000), and mean ± SD release of IFN-γ in duplicate wells is shown.
HLA-DM sensitivity is reversible by HLA-DO. (A) Histograms are shown for class II–associated invariant chain peptide (CLIP) surface expression on MJS wt (open) and MJS cells transduced with HLA-DOα/β (filled) as measured by flow cytometry. (B) The influence of HLA-DO on presentation of the DM-sensitive antigen PI4K2B and DM-resistant antigen PTK2B was analyzed by testing T-cell recognition of MJS wt (open bars) and MJS transduced with HLA-DOα/β (closed bars). MJS melanoma cells endogenously express the invariant chain and HLA-DM but lack expression of HLA-DO. The HLA-DRB3*0101 restriction allele for PTK2B is endogenously expressed by MJS cells and the HLA-DQB1*0603/DQA1*0103 restriction allele for the HLA class II epitope from PI4K2B was introduced retrovirally. To induce antigen expression, MJS cells were loaded with recombinant proteins in 2 different concentrations. Mean ± SD release of IFN-γ in duplicate wells is shown. (C) MJS-DQ6 (open bars) and MJS-DQ6-DO (closed bars) cells were transduced with retroviral vectors encoding PI4K2B and PTK2B to test the influence of HLA-DO on endogenously expressed antigens. T-cell recognition was tested against titrated numbers of target cells (10 000 and 3000), and mean ± SD release of IFN-γ in duplicate wells is shown.
These data indicated that there are 2 distinct subsets of HLA class II–restricted antigens: one that behaves like CLIP and whose presentation is determined by a delicate balance between Ii, HLA-DM, and HLA-DO expression and another set of HLA class II epitopes whose presentation is not or less dependent on this balance.
T cells recognizing DM-sensitive HLA class II epitopes represented a significant part of the natural repertoire
Our data showed that 3 of 6 identified HLA class II epitopes are DM sensitive. We next investigated the overall contribution of DM-sensitive epitopes to natural T-cell responses. For this purpose, we generated and screened CD4+ T-cell libraries according to the method described by Geiger et al.24 Briefly, CD4+ T cells were purified and seeded at 2 000 T cells per well. CD4+ T cells were nonspecifically expanded in the presence of irradiated allogeneic PBMCs, PHA, and IL-2. After 3 weeks of in vitro expansion, all T-cell pools were tested against HeLa variants expressing the Ii with and without cotransduced HLA-DM in IFN-γ ELISA. CD4+ T-cell libraries from 3 different donors were screened against HeLa variants transduced with the HLA class II molecules as expressed by the donors. T-cell pools with no or low (< 200pg/mL IFN-γ) reactivity were scored as “nonreactive,” whereas the remaining pools were separated into DM-resistant or -sensitive phenotypes depending on differential recognition of HeLa-Ii and HeLa-Ii/DM. HLA class II epitopes were considered DM sensitive when recognition in the context of HLA-DM was decreased by more than 50%. The data showed that separation of HLA class II epitopes into DM-resistant and DM-sensitive epitopes was not restricted to the 6 antigens analyzed in more detail but represented a widely occurring phenomenon observed for HLA class II epitopes in natural T-cell responses. Our data furthermore showed that the majority of epitopes recognized in the context of HLA-DQ display a DM-sensitive phenotype (Figure 5A), whereas for HLA-DR alleles a tendency toward more DM-resistant epitopes was observed (Figure 5B).
DM-resistant and DM-sensitive antigens as recognized by the natural CD4+ T-cell repertoire. Purified CD4+ T cells were seeded in one 96-well plate, each well containing 2000 different T cells. CD4+ T cells were nonspecifically expanded and tested for recognition of HeLa–invariant chain (Ii) and HeLa-Ii/DM transduced with different HLA class II molecules. T-cell pools with no or low (< 200 pg/mL IFN-γ) recognition of both HeLa variants are depicted as “nonreactive.” The remaining pools were separated into DM-sensitive and DM-resistant phenotype, based on the difference in recognition between HeLa-Ii and HeLa-Ii/DM. Pools with > 50% decrease in recognition of HeLa-Ii/DM versus HeLa-Ii were assigned DM-sensitive, whereas all other pools were counted as DM-resistant. CD4+ T-cell libraries were generated from 3 different donors, and CD4+ T-cell pools were tested against HeLa-Ii and HeLa-Ii/DM variants transduced with HLA-DQB1*0603/A*0103 (2 donors), DQB1*0501/A*0101 (1 donor), DRB1*0301 (1 donor), DRB1*1301 (2 donors), DRB3*0101 (1 donor), and DRB3*0202 (1 donor). Representative results are shown for 2 HLA-DQ alleles (A) and 4 HLA-DR molecules (B). The total number and percentages of DM-resistant and DM-sensitive pools for each HLA class II allele are indicated.
DM-resistant and DM-sensitive antigens as recognized by the natural CD4+ T-cell repertoire. Purified CD4+ T cells were seeded in one 96-well plate, each well containing 2000 different T cells. CD4+ T cells were nonspecifically expanded and tested for recognition of HeLa–invariant chain (Ii) and HeLa-Ii/DM transduced with different HLA class II molecules. T-cell pools with no or low (< 200 pg/mL IFN-γ) recognition of both HeLa variants are depicted as “nonreactive.” The remaining pools were separated into DM-sensitive and DM-resistant phenotype, based on the difference in recognition between HeLa-Ii and HeLa-Ii/DM. Pools with > 50% decrease in recognition of HeLa-Ii/DM versus HeLa-Ii were assigned DM-sensitive, whereas all other pools were counted as DM-resistant. CD4+ T-cell libraries were generated from 3 different donors, and CD4+ T-cell pools were tested against HeLa-Ii and HeLa-Ii/DM variants transduced with HLA-DQB1*0603/A*0103 (2 donors), DQB1*0501/A*0101 (1 donor), DRB1*0301 (1 donor), DRB1*1301 (2 donors), DRB3*0101 (1 donor), and DRB3*0202 (1 donor). Representative results are shown for 2 HLA-DQ alleles (A) and 4 HLA-DR molecules (B). The total number and percentages of DM-resistant and DM-sensitive pools for each HLA class II allele are indicated.
HLA-DQ and HLA-DOβ are predominantly expressed in B cells and mature DCs
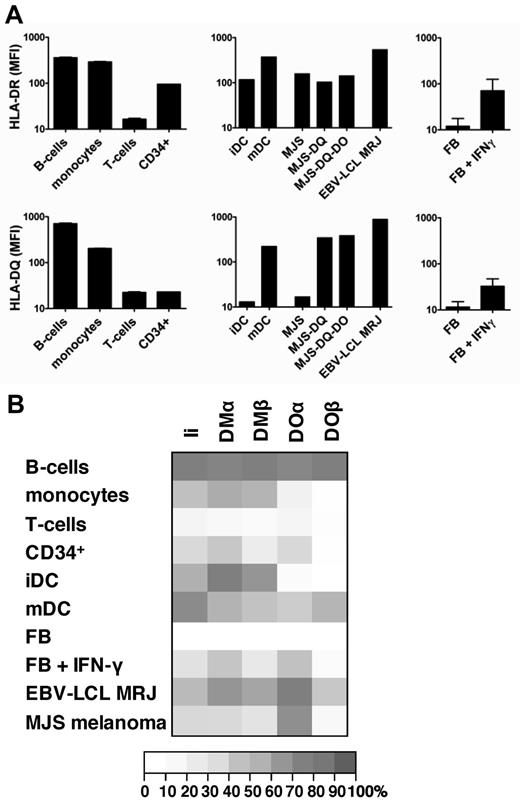
Our data found that presentation of DM-sensitive epitopes depended on balanced expression of Ii, HLA-DM, and HLA-DO. Because most of the natural HLA class II epitopes presented by HLA-DQ are DM sensitive, we analyzed the surface expression patterns of HLA-DR and -DQ in more detail by flow cytometry. In addition, expression of the genes encoding Ii, HLA-DMα/β, and HLA-DOα/β was evaluated in hematopoietic cells and fibroblasts. To investigate the effect of IFN-γ on the HLA class II machinery in nonhematopoietic cells, fibroblasts were analyzed with and without pretreatment with IFN-γ. HLA-DR was strongly expressed on B cells, monocytes, CD34+ cells, immature and mature DCs, and IFN-γ–treated fibroblasts, whereas no HLA-DR expression was observed on T cells and nontreated fibroblasts. In contrast, HLA-DQ expression was high on B cells and mature DCs but significantly lower than HLA-DR on monocytes, immature DCs, CD34+ cells, and IFN-γ–treated fibroblasts (Figure 6A). This indicated that HLA-DQ was preferentially expressed on professional APCs, including B cells and mature DCs. Expression analysis of the genes encoding Ii, HLA-DMα/β, and HLA-DOα/β by microarray gene expression profiling revealed lack or only weak expression in HLA class II–negative T cells and nontreated fibroblasts. In contrast, all HLA-DR–positive cells, including monocytes, B cells, immature and mature DCs, CD34+ cells, and IFN-γ–treated fibroblasts, showed gene expression for Ii, HLA-DMα/β, and HLA-DOα, albeit at different levels. However, different patterns of gene expression were observed for HLA-DOβ. Whereas HLA-DOβ was strongly expressed in B cells and mature DCs, no significant expression could be measured in the other cell types (Figure 6B). The data therefore showed that HLA-DQ and HLA-DOβ are coexpressed on professional APCs, including B cells and mature DCs, suggesting that these cells may represent the subset of HLA class II–positive cells capable of inducing CD4+ T cells against DM-sensitive antigens.
Coordinated expression of HLA-DQ and HLA-DOβ in professional APCs. (A) The mean fluorescence intensity (MFI) of flow cytometric staining for HLA-DR (left) and HLA-DQ (right) are shown for primary B cells (CD19+), monocytes (CD14+), T cells (CD3+), hematopoietic stem cells (CD34+), immature and mature dendritic cells (DCs), MJS melanoma cells with and without transduced HLA-DQ and HLA-DO, EBV-transformed B-cell lines (EBV-LCL) from patient MRJ, as well as fibroblasts with and without IFN-γ treatment. For primary cells means and SDs of duplicate stainings are shown. (B) mRNA expression of invariant chain (Ii), DMα and DMβ, and DOα and DOβ was measured by microarray gene expression analysis in primary B cells, monocytes, T cells, hematopoietic stem cells (CD34+), immature and mature DCs, MJS melanoma cells, EBV-LCL from patient MRJ, as well as fibroblasts with and without IFN-γ treatment. Relative gene expression is depicted for the mean of 3 independent samples for each cell type.
Coordinated expression of HLA-DQ and HLA-DOβ in professional APCs. (A) The mean fluorescence intensity (MFI) of flow cytometric staining for HLA-DR (left) and HLA-DQ (right) are shown for primary B cells (CD19+), monocytes (CD14+), T cells (CD3+), hematopoietic stem cells (CD34+), immature and mature dendritic cells (DCs), MJS melanoma cells with and without transduced HLA-DQ and HLA-DO, EBV-transformed B-cell lines (EBV-LCL) from patient MRJ, as well as fibroblasts with and without IFN-γ treatment. For primary cells means and SDs of duplicate stainings are shown. (B) mRNA expression of invariant chain (Ii), DMα and DMβ, and DOα and DOβ was measured by microarray gene expression analysis in primary B cells, monocytes, T cells, hematopoietic stem cells (CD34+), immature and mature DCs, MJS melanoma cells, EBV-LCL from patient MRJ, as well as fibroblasts with and without IFN-γ treatment. Relative gene expression is depicted for the mean of 3 independent samples for each cell type.
DM-sensitive antigens are efficiently presented on mature DCs
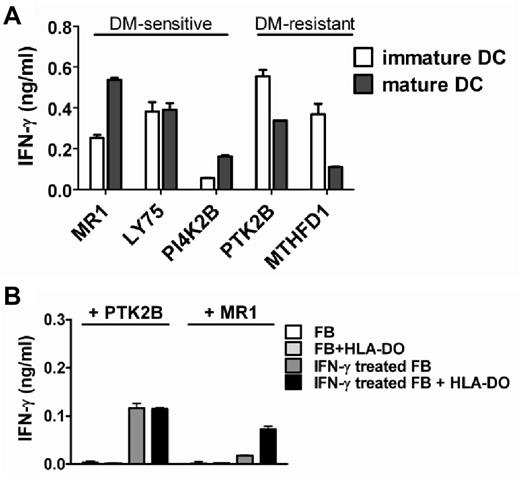
Because HLA-DMα/β gene expression was diminished and HLA-DOα/β gene expression was strongly induced on DC maturation, we hypothesized that mature DCs may be particularly relevant as primary cell type for in vivo presentation of DM-sensitive antigens, whereas DM-resistant antigens may also be efficiently presented on immature DCs. To investigate this, we tested T-cell recognition of immature and mature DCs generated from monocytes of patient MRJ cells that expressed all 5 antigens and their respective HLA class II restriction alleles. The data confirmed that presentation of DM-sensitive antigens benefited more from maturation of DCs compared with DM-resistant antigens (Figure 7A)
Presentation of DM-sensitive antigens on primary hematopoietic and IFN-γ–treated nonhematopoietic cell types is regulated by HLA-DO. (A) Monocyte-derived immature (open bars) and mature (closed bars) dendritic cells (DCs) were generated from patient MRJ expressing all 5 antigens as well as their respective HLA class II restriction alleles, and recognition by the specific T-cell clones was tested by IFN-γ ELISA. Indicated is the T-cell recognition of mature versus immature DCs. (B) Primary fibroblasts expressing HLA-DRB3*0101 and DRB3*0202 with and without transduced HLA-DO were pulsed with recombinant MR1 (DM-sensitive) and PTK2B (DM-resistant) proteins, and recognition by the specific T-cell clones was tested without cytokine pretreatment or after 3-4 days of treatment with IFN-γ. Mean ± SD release of IFN-γ in duplicate wells is shown. The fibroblasts used for T-cell recognition were included in the microarray analysis as depicted in Figure 6B.
Presentation of DM-sensitive antigens on primary hematopoietic and IFN-γ–treated nonhematopoietic cell types is regulated by HLA-DO. (A) Monocyte-derived immature (open bars) and mature (closed bars) dendritic cells (DCs) were generated from patient MRJ expressing all 5 antigens as well as their respective HLA class II restriction alleles, and recognition by the specific T-cell clones was tested by IFN-γ ELISA. Indicated is the T-cell recognition of mature versus immature DCs. (B) Primary fibroblasts expressing HLA-DRB3*0101 and DRB3*0202 with and without transduced HLA-DO were pulsed with recombinant MR1 (DM-sensitive) and PTK2B (DM-resistant) proteins, and recognition by the specific T-cell clones was tested without cytokine pretreatment or after 3-4 days of treatment with IFN-γ. Mean ± SD release of IFN-γ in duplicate wells is shown. The fibroblasts used for T-cell recognition were included in the microarray analysis as depicted in Figure 6B.
DM-sensitive antigens are inefficiently presented on cytokine-treated nonhematopoietic cells because of lack of HLA-DO
Furthermore, in contrast to Ii, HLA-DMα/β, and HLA-DOα, we could confirm earlier reports that expression of HLA-DOβ is hardly induced in nonhematopoietic cells on IFN-γ treatment. This suggested that, even under inflammatory conditions, DM-sensitive antigens are not or inefficiently presented on nonhematopoietic cells. We here tested processing and presentation of a DM-sensitive (MR1) and DM-resistant (PTK2B) antigen in skin-derived fibroblasts endogenously expressing the relevant HLA-DR restriction alleles. Fibroblasts with and without IFN-γ treatment were pulsed with recombinant proteins to induce antigen expression and were tested for T-cell recognition. In line with the absence of HLA-DR expression, untreated fibroblasts were not recognized by the respective T-cell clones (Figure 7B). After up-regulation of HLA class II by IFN-γ treatment, the DM-resistant antigen PTK2B was clearly recognized, whereas the DM-sensitive antigen MR1 hardly induced T-cell recognition. Introduction of HLA-DO enhanced presentation of DM-sensitive antigen MR1, whereas it did not affect presentation of DM-resistant antigen PTK2B. In conclusion, the data confirm that DM-sensitive antigens are inefficiently presented on cytokine-treated nonhematopoietic cells because of lack of HLA-DO.
Discussion
CD4+ T cells play a key role in adaptive immunity. They promote induction and maintenance of CD8+ T cells and induce humoral immunity.2,3 In addition, they can function as potent cytolytic effector cells.4–7 According to this central role in the immune system, presentation of HLA class II epitopes to CD4+ T cells is tightly regulated, a task attributed to the peptide editing function of the nonclassic HLA class II molecule HLA-DM.28–30 Here, we show that endogenous HLA class II epitopes that are immunogenic in vivo consist of 2 groups. One group of antigens is presented on all HLA class II–positive cells (DM-resistant), whereas the other group displays a CLIP-like behavior and their presentation depends on a delicate balance between Ii, HLA-DM, and HLA-DO (DM-sensitive).
It is well established that HLA-DM promotes the removal of CLIP, and this is believed to be necessary for efficient presentation of antigenic peptides.29,30 Surface expression of CLIP has therefore been regarded as a marker for defective antigen presentation. Here, we demonstrate that 3 of 6 natural HLA class II epitopes that were recognized by CD4+ T cells in in vivo immune responses display a similar behavior toward HLA-DM as CLIP. Moreover, in our cell line models, surface expression of CLIP, either because of absence of HLA-DM or coexpression of HLA-DO, correlated with presentation of a broad peptide repertoire. The existence of DM-sensitive antigens as a relevant category of HLA class II epitopes has been further confirmed by our T-cell library experiments as well as an earlier mouse study,31 showing that at least a quarter of undefined self-antigens in natural T-cell responses are recognized in a DM-sensitive manner. These data are, however, in striking contrast with the general assumption that HLA-DM is necessary to promote presentation of bona fide HLA class II epitopes. A possible explanation for this contradiction might be the origin of the presented epitope. Most studies have focused on exogenous antigens, whereas we here analyzed presentation of endogenous proteins. It has been shown by biochemical studies31–34 that HLA-DM promotes the formation of long-lived HLA-peptide complexes.35 This should ensure that exogenous antigens, which are taken up and processed by DCs in the periphery, are presented sufficiently long to allow migration of the DCs to the draining lymph node and to prime naive T cells.36 The requirement for long-lived HLA-peptide complexes may, however, be less stringent for endogenous antigens because of continuous assembly and export of intracellular HLA-peptide complexes to the cell surface.37 This may explain why T-cell responses against endogenous antigens are not skewed toward DM-resistant epitopes but also allow recognition of DM-sensitive antigens which are exclusively intracellularly processed (supplemental Figure 3). HLA-DM is supposed to mediate peptide exchange on interaction with HLA class II molecules, and the global conformation of a HLA-peptide complex enables or disrupts interaction with HLA-DM. Mutagenesis studies found that the lateral surface of HLA-DM and HLA-DR are important for the DM-DR interaction.25,38 In line with this, mutation of residues E40 and F51 in the DRα chain completely alleviated the inhibitory effect of HLA-DM on presentation of our DM-sensitive epitopes. These residues were previously found to be necessary for HLA-DM–mediated removal of CLIP,25 further supporting the CLIP-like behavior of our DM-sensitive epitopes. Mutations in the DRα chain, however, did not significantly alter presentation of DM-resistant antigens. Thus, although others have postulated that the function of HLA-DM is to keep the HLA restriction molecule in an “open, peptide-receptive” conformation,39 our data support an “open, peptide-exchange” configuration in which HLA-DM stimulates peptide release until a peptide binds that induces a conformational change in the HLA restriction molecule that disrupts interaction with HLA-DM.
We demonstrated that presentation of DM-sensitive epitopes could be restored by HLA-DO. This nonclassic class II molecule is selectively expressed in B cells, DCs, and thymic epithelial cells,19,40 and, although it is known to alter the presented peptide repertoire by interacting with HLA-DM, its biologic function is unclear. Mice lacking H2-O (murine HLA-DO) show impaired ability to generate humoral immune responses with slow and reduced antibody responses, but, when growing older, these mice spontaneously develop high titers of autoantibodies. This contradictory phenotype indicates a dual role for HLA-DO. Because we here show that a broad repertoire of endogenous peptides is presented in the presence of HLA-DO, it can be speculated that expression of HLA-DO in professional APCs may be relevant for inducing a broad T-helper response.41,42 If true, slow and reduced antibody responses in mice lacking H2-O may reflect impaired T-cell help against exogenous antigens. HLA-DO expression in thymic epithelial cells, however, might be necessary for central tolerance by inducing apoptosis or anergy of T cells against a maximum repertoire of self-peptides. In T-cell library experiments, we noticed a dominance of DM-sensitive antigens in HLA-DQ. Because HLA-DQ and HLA-DO are coordinately expressed in professional APCs, it can be assumed that DM-sensitive antigens in HLA-DQ may be particularly relevant in the onset of immune responses to induce a broad CD4+ T-helper response without stimulating reactivity against HLA class II–positive cells lacking HLA-DO.
The restricted expression profile of HLA-DO and its influence on the presented peptide repertoire implicates that there are 2 types of HLA class II–positive cells, presenting overlapping but distinct sets of peptides. This is also supported by peptide elutions from B cells and melanoma cell lines with and without transfected HLA-DO, indicating that a fraction of peptides is specific for HLA-DO–expressing cells (10%-26%).20,43,44 In line with this, we could show that lack of HLA-DO in cell lines and primary fibroblasts precludes efficient presentation of DM-sensitive antigens. HLA-DO expression might therefore explain earlier observations that certain cell types are not recognized by CD4+ T-cell clones despite substantial expression of HLA class II and the antigen. We noticed that the T-cell clones for DM-sensitive antigens MR1 and LY75 did not recognize patient-derived CD34+ chronic myelogenous leukemia progenitor cells,14 which is in line with low HLA-DO expression in these cells. Others showed that 2 CD4+ T-cell clones against MELOE-1 failed to recognize melanoma cells.15 Because the melanoma cells expressed sufficient antigen and HLA class II, it is tempting to speculate that the antigens are DM-sensitive epitopes that are inefficiently presented because of lack of HLA-DO.
To exploit adaptive immunity against cancer, the existence of 2 types of HLA class II–expressing cells presenting different peptide profiles based on expression of HLA-DO opens an interesting perspective. In alloSCT donor-derived T cells recognize and eradicate patient-derived malignant cells, thereby mediating beneficial GVL effect.45 These T cells, however, may also react against healthy nonhematopoietic tissues of the patient, thereby inducing GVHD, the main cause of morbidity and mortality.46 Many efforts have been directed toward separation of the GVL effect and GVHD, and clinical data indicate that these 2 phenomena can indeed arise independently.6 One option to shift the balance toward GVL effect is to exploit CD4+ T cells as HLA class II expression is mainly restricted to cells of the hematopoietic system. The main drawback of this approach is that nonhematopoietic cells up-regulate HLA class II under inflammatory conditions which often occur in the setting of alloSCT because of the conditioning regimen or (viral) infections,46 and this may trigger CD4+ T cells to cause GVHD.
We show that DM-sensitive antigens are not efficiently presented on cytokine-treated nonhematopoietic cells because of lack of up-regulation of HLA-DOβ by IFN-γ.47 On the basis of these data, T cells recognizing DM-sensitive antigens are not expected to mediate GVHD. Intriguingly, our preliminary experiments found broad expression of HLA-DO in primary leukemic cells of different origins. T-cell therapy targeting DM-sensitive antigens may therefore allow selective eradication of DO-expressing malignant cells with reduced risk of GVHD. Because CLIP-expressing leukemias have been reported to exhibit a particularly bad prognosis,48 specific targeting of DM-sensitive antigens with CLIP-like behavior may have additional therapeutic value.
In conclusion, our detailed analysis of processing and presentation of natural HLA class II epitopes provides novel insights into the relative roles of HLA-DM and -DO in presentation of endogenous antigens. The findings underline that, in addition to HLA class II and antigen expression, antigen processing should be considered as a main factor for optimal use and development of CD4+ T cell–based immunotherapy. Furthermore, these data might lay the basis for a new approach of targeted therapy that is not based on selective antigen expression but on selective processing capacities of the target cells.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank M. van der Hoorn, G. de Roo, and P. van der Holst for technical assistance with flow cytometric isolation.
This work was supported by the Dutch Cancer Society (UL2008-4111) and the German Research Foundation (DFG; STU540/1-1).
Authorship
Contribution: A.N.K., J.H.F.F., and M.G. designed research; A.N.K., E.D.v.d.M., M.W.H., and M.G. performed research; A.N.K., M.W.H., J.J.G., J.H.F.F., and M.G. analyzed data; and A.N.K., E.J.H.J.W., J.H.F.F., and M.G. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anita N. Kremer, Department of Hematology, Leiden University Medical Center, PO Box 9600, 2300 RC, Leiden, The Netherlands; e-mail: anita.kremer@aol.de.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal