Abstract
We here investigate the occurrence of fluoride intake-associated alterations in patients with hematologic disease on triazol antifungal medication. Clinical, laboratory, and radiology data of overall 43 patients with hematologic malignancies taking voriconazole (n = 20), posaconazole (n = 8), and itraconazole (n = 4), and a hematologic patient control group (n = 11) are described. Bone pain and radiologic evidence of periostitis were exclusively observed in patients receiving long-term voriconazole. Cessation of treatment led to clinical improvement in all cases. In line with clinical evidence, fluoride serum concentration was elevated in patients receiving voriconazole (median, 156.5 μg/L; interquartile range, 96.8 μg/L; normal < 30 μg/L) but not in the other treatment groups (P < .001 for all comparisons vs voriconazole). We conclude that serum fluoride levels were elevated on average 5-fold above normal levels in hematologic patients receiving voriconazole. Clinically relevant skeletal disease was associated with renal insufficiency and above 10-fold elevated fluoride levels, and was reversible on termination of voriconazole treatment.
Introduction
Invasive fungal infections are an important cause of morbidity and mortality among patients with hematologic malignancies undergoing intensive chemotherapy with or without autologous or allogeneic hematopoietic stem cell transplantation.1 The incidence of invasive aspergillosis in this patient population ranges from 4% to 12%.2 In most patients, the diagnosis of invasive aspergillosis triggers prolonged antifungal treatment. Voriconazole, a fluorinated triazole compound, is an established first-line treatment for invasive aspergillosis and is recommended by most international guidelines.3,4 Its most common side effects are well known and include visual disturbances, hallucinations, edema, hepatotoxicity, phototoxicity, cutaneous carcinogenesis, and drug interactions resulting from cytochrome p450 inhibition.5 In addition, recent case reports of periostitis and skeletal disease in solid organ recipients with long-term voriconazole treatment have been published.6-8 It was suggested that the fluorinated moieties of voriconazole might play a pathogenetic role, as the radiologic picture resembled that of “subacute fluorosis.” However, up to now, only 1 study assessed fluoride levels in a small cohort (n = 10) of primarily solid transplant patients, including only 1 patient after bone marrow transplantation.9 Furthermore, the effect of posaconazole, a newer fluorinated triazole drug, on serum fluoride levels is unknown.
We here describe fluoride levels, renal function, and musculoskeletal symptoms in a cohort of hematologic patients receiving treatment with voriconazole, posaconazole, or itraconazole and compare these with similar hematologic patients receiving no antifungal therapy.
Methods
Patients and clinical data
We report 3 patients with bone pain under antifungal therapy with voriconazole and an additional cohort of hematologic patients (n = 43) treated with antifungal therapy between June 2011 and August 2011 at the Division of Hematology, University Hospital Zurich. Inclusion criteria were a history of intensive chemotherapy and/or allogeneic stem cell transplantation. We included all patients treated with voriconazole, itraconazole, and posaconazole and a control group that was treated during the same time but did not receive antifungal treatment. Clinical information was extracted from the patient's charts. Bone pain was assessed by patient interview on the day of fluoride measurement. The retrospective study was approved by the local ethical committee of the Canton Zurich, Switzerland.
Radiologic assessment and laboratory measurements
The presence of skeletal disease was assessed by 2 independent radiologists who were blinded for the patient's clinical data. Radiographic signs of periosteal reaction and skeletal disease consisted of periosteal thickening, periosteal calcification, calcification of ligaments, and osteosclerosis. Discordant interpretations were resolved by consensus in a second readout. The analysis included the conventional radiographs (n = 60) and CT scans and low-dose CT scans (n = 67) taken between January 2011 and August 2011. In patients with fungal infections only, radiologic studies taken after initiation of the antifungal treatment were included.
Fluoride levels were determined from patient serum and measured by potentiometry with an ion-selective electrode (Mettler Toledo, SevenMulti). Laboratory values were assessed on the date of fluoride measurement, with the exception of drug serum levels, which were allowed to be assessed during a time frame of 7 days before or after the fluoride measurement took place. Drug levels were measured by HPLC mass spectrometry (Finnigan TSQ LC/MS) at baseline.
The glomerular filtration rate was calculated according the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula.
Definition of medication-induced skeletal disease
Diagnosis of medication-induced skeletal disease required (1) typical clinical findings, such as a subacute disseminated musculoskeletal pain syndrome; (2) radiographic findings, such as periosteal appositions or disseminated focal tracer uptake in a bone scintigraphy; (3) absence of an alternative diagnosis, such as relapse, secondary cancer, and arthritis; and (4) prompt improvement after drug cessation.
Statistical analysis
Data were reported as proportions, means with SDs, or medians with interquartile ranges (IQRs). Fluoride levels were compared by the Kruskal-Wallis test. All reported P values are 2-sided, and P < .05 was considered to be significant. The Spearman rank correlation coefficient was used to estimate the correlation between the serum fluoride level and the glomerular filtration rate.
Results
Case descriptions
We report 3 patients on long-term voriconazole treatment who developed clinically significant skeletal disease, which was completely reversible after termination of voriconazole (Table 1). All 3 patients were female allogeneic stem cell transplant recipients with cyclosporine-related moderate chronic renal failure. Disseminated bone pain started between 3 and 7.5 months of voriconazole treatment. Laboratory findings included an elevated alkaline phosphatase and bone-specific alkaline phosphatase, normal levels of calcium, phosphate, parathyroid hormone, 25-hydroxyvitamin D, and a slightly elevated urinary deoxypyridinoline/creatinine quotient. Conventional radiographs, CT scans, and bone scintigraphy revealed periosteal appositions and focal tracer uptake, respectively (Figures 1 and 2). CT scans of the chest and abdomen showed no signs of secondary malignancies, and bone marrow biopsies ruled out recurrence of the acute leukemia. Patients 1 and 2 were initially misdiagnosed as having an unusual musculoskeletal presentation of GVHD; hence, the immunosuppression was intensified. However, the pain was only temporarily attenuated by corticosteroids and methotrexate but disappeared almost completely in all 3 patients within 4 days of voriconazole cessation. The diagnosis of voriconazole-induced skeletal disease was made only retrospectively in patient 1, 20 months after occurrence of the first symptoms in patient 2, and without delay in patient 3. Serum fluoride measurements at diagnosis were available for patients 2 and 3 with > 10-fold elevated levels and a marked decrease within 3 weeks of voriconazole cessation (81% and 57% of precessation values, respectively). Long-term follow up of 5.5 years after the initial diagnosis revealed complete resolution of the skeletal changes in patient 1 (Figure 1).
Clinical and laboratory characteristics of patients with voriconazole-induced skeletal disease
| . | Patient 1 . | Patient 2 . | Patient 3 . |
|---|---|---|---|
| Diagnosis | AML | AML | ALL |
| Age at diagnosis, y | 46 | 37 | 55 |
| Sex | Female | Female | Female |
| Body mass index, kg/m2 | 17.5 | 24.4 | 19.4 |
| Allogeneic stem cell transplantation | |||
| Conditioning | Cy/TBI | Cy/TBI | Cy/TBI |
| Donor | MSD | MSD | MSD |
| GVHD prophylaxis | CsA/MTX | CsA/MTX | CsA/MTX |
| Voriconazole treatment days until onset of skeletal disease | 100 | 177 | 226 |
| Total voriconazole dose until onset of skeletal disease, g | 55 | 97.4 | 133.6 |
| Median voriconazole level (IQR)*/no. of measurements | 1.6 (0.85)/12 | 1.95 (0.9)/14 | 1.2 (1.35)/15 |
| Laboratory values during voriconazole treatment/3 wks after voriconazole cessation | |||
| Glomerular filtration rate, mL/min† | 57/65 | 31/50 | 32/46 |
| ALP, U/L‡ | 195/102 | 384/214 | 202/125 |
| ALT, U/L§ | 106/92 | 38/30 | 9/13 |
| Fluoride level, μg/L‖ | NA | 363/70 | 316/136 |
| CsA level, μg/L | 154/NA | 106/50 | 226/179 |
| . | Patient 1 . | Patient 2 . | Patient 3 . |
|---|---|---|---|
| Diagnosis | AML | AML | ALL |
| Age at diagnosis, y | 46 | 37 | 55 |
| Sex | Female | Female | Female |
| Body mass index, kg/m2 | 17.5 | 24.4 | 19.4 |
| Allogeneic stem cell transplantation | |||
| Conditioning | Cy/TBI | Cy/TBI | Cy/TBI |
| Donor | MSD | MSD | MSD |
| GVHD prophylaxis | CsA/MTX | CsA/MTX | CsA/MTX |
| Voriconazole treatment days until onset of skeletal disease | 100 | 177 | 226 |
| Total voriconazole dose until onset of skeletal disease, g | 55 | 97.4 | 133.6 |
| Median voriconazole level (IQR)*/no. of measurements | 1.6 (0.85)/12 | 1.95 (0.9)/14 | 1.2 (1.35)/15 |
| Laboratory values during voriconazole treatment/3 wks after voriconazole cessation | |||
| Glomerular filtration rate, mL/min† | 57/65 | 31/50 | 32/46 |
| ALP, U/L‡ | 195/102 | 384/214 | 202/125 |
| ALT, U/L§ | 106/92 | 38/30 | 9/13 |
| Fluoride level, μg/L‖ | NA | 363/70 | 316/136 |
| CsA level, μg/L | 154/NA | 106/50 | 226/179 |
AML indicates acute myeloid leukemia; ALL, acute lymphoblastic leukemia; Cy/TBI, cyclophosphamide/total body irradiation (12 Gy); MSD, HLA-matched sibling donor; CsA/MTX, cyclosporine/methotrexate; ALP, alkaline phosphatase; ALT, alanine aminotransferase; and NA, not applicable.
Target serum levels for voriconazole 1.0-6.0 mg/L, posaconazole > 1.0 mg/L, and itraconazole > 1.0 mg/L.
According to the CKD-EPI formula.
Normal: 35-104 U/L.
Normal: 10-35 U/L.
Normal: < 30 μg/L.
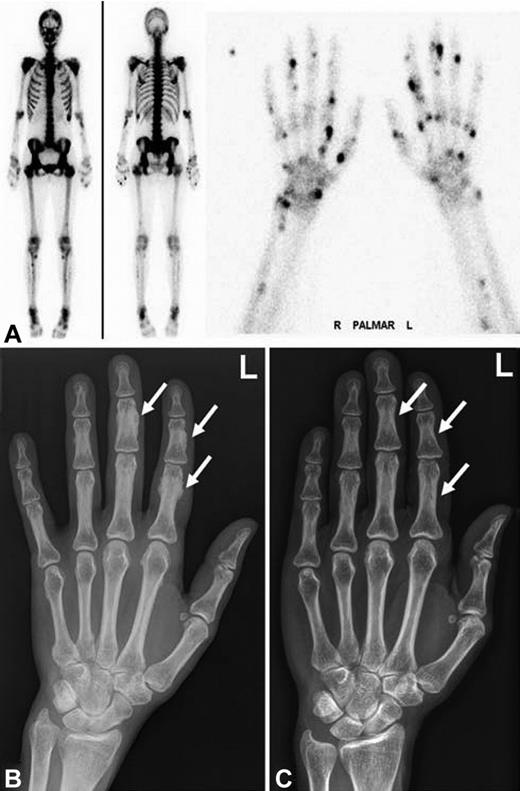
Radiographic findings and bone scintigraphy in patient 1. (A) Technetium 99m-methyl-diphosphonate bone scintigraphy showing marked tracer uptake in the entire skeleton, most pronounced in the spine and pelvis (left). There are multiple active spots in bones of both hands (right). (B) Plain film showing marked periosteal bone apposition on diaphysis of the phalanges of the left hand (arrows). Findings are typical for hypertrophic osteoarthropathy. The image was taken after 9 months of voriconazole therapy. (C) Plain film showing resolution of periosteal bone appositions on diaphysis of the phalanges (arrows). The image was taken 5 years after cessation of voriconazole therapy.
Radiographic findings and bone scintigraphy in patient 1. (A) Technetium 99m-methyl-diphosphonate bone scintigraphy showing marked tracer uptake in the entire skeleton, most pronounced in the spine and pelvis (left). There are multiple active spots in bones of both hands (right). (B) Plain film showing marked periosteal bone apposition on diaphysis of the phalanges of the left hand (arrows). Findings are typical for hypertrophic osteoarthropathy. The image was taken after 9 months of voriconazole therapy. (C) Plain film showing resolution of periosteal bone appositions on diaphysis of the phalanges (arrows). The image was taken 5 years after cessation of voriconazole therapy.
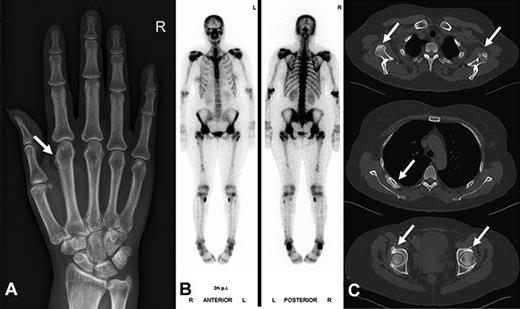
Radiographic findings and bone scintigraphy in patient 2. (A) Plain film showing marked periosteal bone apposition on radial and distal diaphysis of the first metacarpal bone of the right hand (arrow). (B) Technetium 99m-methyl-diphosphonate bone scintigraphy showing marked tracer uptake in the entire skeleton, most pronounced in the spine and pelvis. There are active spots in bones of both hands and feet. (C) CT scan of the entire skeleton revealed bone appositions in various sites of the skeleton (arrows): at the caudal margin of the glenoid (top), at the dorsal ribs (middle), and periacetabular on both sides (bottom). Findings are typical of hypertrophic osteoarthropathy.
Radiographic findings and bone scintigraphy in patient 2. (A) Plain film showing marked periosteal bone apposition on radial and distal diaphysis of the first metacarpal bone of the right hand (arrow). (B) Technetium 99m-methyl-diphosphonate bone scintigraphy showing marked tracer uptake in the entire skeleton, most pronounced in the spine and pelvis. There are active spots in bones of both hands and feet. (C) CT scan of the entire skeleton revealed bone appositions in various sites of the skeleton (arrows): at the caudal margin of the glenoid (top), at the dorsal ribs (middle), and periacetabular on both sides (bottom). Findings are typical of hypertrophic osteoarthropathy.
Results of the cohort with hematologic malignancies receiving antifungal therapy and the control group
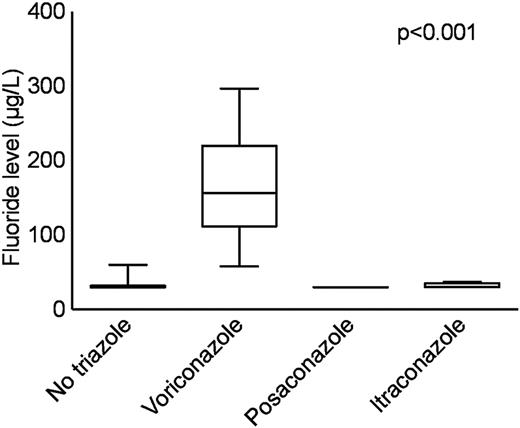
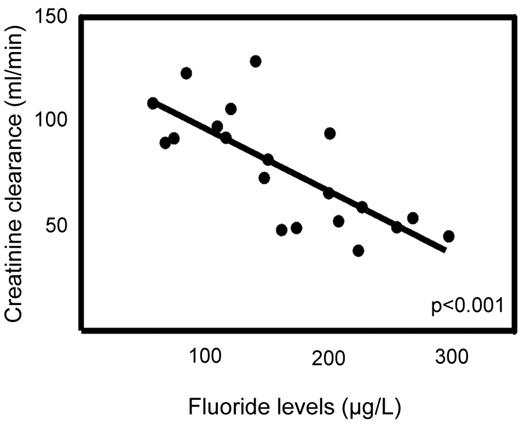
The main patient characteristics of overall 32 patients with hematologic malignancies taking voriconazole (n = 20), posaconazole (n = 8), and itraconazole (n = 4), as well as a hematologic patient control group (n = 11) not receiving antifungal therapy are shown in Table 2 and in more detail in Supplemental Tables 1 and 2 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The 3 patients with proven voriconazole-induced skeletal disease were not included in this analysis. Serum fluoride levels were significantly elevated in patients receiving voriconazole (median, 157 μg/L; IQR, 97 μg/L) compared with itraconazole (median, < 30 μg/L; IQR, 28 μg/L), posaconazole (median, < 30 μg/L; IQR, 0 μg/L), and the control group without antifungal medication (median, < 30 μg/L; IQR, 28 μg/L; P < .001 for all comparisons; Figure 3). The fluoride level in the voriconazole treatment group was inversely correlated to the glomerular filtration rate (Spearman rank correlation coefficient = 0.74; P < .001; Figure 4). Radiologically typical skeletal disease (periosteal appositions, abnormal calcifications) was seen in the CT scan of 1 asymptomatic patient (5%) in the voriconazole group and none in the other treatment groups. Disseminated bone pain consistent with skeletal disease was present in 3 (15%) patients in the voriconazole group and none in the other treatment groups. No signs of skeletal disease were detected in 2 of these patients by chest CT scan, whereas the third patient had no radiologic examinations performed during the observation phase. Additional fluorinated drugs were given in only 1 patient (posaconazole group), and no increase in serum fluoride level was noted.
Clinical and laboratory characteristics of patients with hematologic malignancies taking voriconazole, posaconazole, and itraconazole, and a hematologic patient control group not receiving antifungal therapy
| . | Voriconazole (n = 20) . | Posaconazole (n = 8) . | Itraconazole (n = 4) . | Control group (n = 11) . |
|---|---|---|---|---|
| Mean age, y | 55.1 | 54.9 | 49.5 | 56.6 |
| Male sex, % | 10 (50) | 3 (37.5) | 3 (75) | 7 (63.6) |
| Stem cell transplantation, % | 15 (75) | 3 (37.5) | 4 (100) | 10 (90.9) |
| Median antifungal treatment, days (IQR) | 98 (220) | 13 (576) | 587 (1400) | NA |
| Median drug serum concentration (IQR)* | 1.7 (1.8) | 0.24 (1.17) | 1.95 (1.75) | NA |
| Median glomerular filtration rate (IQR)† | 77.5 (43.6) | 92.2(33.8) | 70.8 (45.7) | 74.2 (18.9) |
| Cyclosporine treatment, % | 9 (45) | 2 (25) | 0 | 6 (54.5) |
| Median cyclosporine blood concentration, μg/L (IQR) | 92 (102.5) | 53.5 | NA | 104 (141.5) |
| Median ALP‡ (IQR) | 111 (76.5) | 66 (103) | 120 (138) | 71 (31) |
| Median ALT§ (IQR) | 30 (12.5) | 36 (30) | 70.5 (90) | 20 (18) |
| Median fluoride level‖ (IQR) | 156.5 (96.8) | < 30 (0) | < 30 (28) | < 30 (32) |
| Median no. of radiographic studies (IQR) | 2 (3.5) | 0.5 (1) | 1 (0.25) | 3 (3.5) |
| Median no. of CT scans (IQR) | 1 (1) | 0.5 (1) | 1 (0.25) | 2 (2) |
| Radiographic evidence of periostitis, % | 1 (5) | 0 | 0 | 0 |
| Bone pain, % | 3 (15) | 0 | 0 | 0 |
| . | Voriconazole (n = 20) . | Posaconazole (n = 8) . | Itraconazole (n = 4) . | Control group (n = 11) . |
|---|---|---|---|---|
| Mean age, y | 55.1 | 54.9 | 49.5 | 56.6 |
| Male sex, % | 10 (50) | 3 (37.5) | 3 (75) | 7 (63.6) |
| Stem cell transplantation, % | 15 (75) | 3 (37.5) | 4 (100) | 10 (90.9) |
| Median antifungal treatment, days (IQR) | 98 (220) | 13 (576) | 587 (1400) | NA |
| Median drug serum concentration (IQR)* | 1.7 (1.8) | 0.24 (1.17) | 1.95 (1.75) | NA |
| Median glomerular filtration rate (IQR)† | 77.5 (43.6) | 92.2(33.8) | 70.8 (45.7) | 74.2 (18.9) |
| Cyclosporine treatment, % | 9 (45) | 2 (25) | 0 | 6 (54.5) |
| Median cyclosporine blood concentration, μg/L (IQR) | 92 (102.5) | 53.5 | NA | 104 (141.5) |
| Median ALP‡ (IQR) | 111 (76.5) | 66 (103) | 120 (138) | 71 (31) |
| Median ALT§ (IQR) | 30 (12.5) | 36 (30) | 70.5 (90) | 20 (18) |
| Median fluoride level‖ (IQR) | 156.5 (96.8) | < 30 (0) | < 30 (28) | < 30 (32) |
| Median no. of radiographic studies (IQR) | 2 (3.5) | 0.5 (1) | 1 (0.25) | 3 (3.5) |
| Median no. of CT scans (IQR) | 1 (1) | 0.5 (1) | 1 (0.25) | 2 (2) |
| Radiographic evidence of periostitis, % | 1 (5) | 0 | 0 | 0 |
| Bone pain, % | 3 (15) | 0 | 0 | 0 |
NA indicates not applicable; ALP, alkaline phosphatase; and ALT, alanine aminotransferase.
Target serum levels for voriconazole 1.0-6.0 mg/L, posaconazole > 1.0 mg/L, and itraconazole > 1.0 mg/L.
According to the CKD-EPI formula.
Normal: 35-104 U/L.
Normal: 10-35 U/L.
Normal: < 30 μg/L.
Serum fluoride levels. The boxplots represent median, IQR, and the whiskers, 95% confidence intervals. Serum fluoride levels were significantly elevated in patients receiving voriconazole (median, 157 μg/L; IQR, 97 μg/L) compared with itraconazole (median < 30 μg/L; IQR, 28 μg/L), posaconazole (median < 30 μg/L, IQR, 0 μg/L), and the control group without antifungal medication (median < 30 μg/L; IQR, 28 μg/L). P < .001 for all comparisons.
Serum fluoride levels. The boxplots represent median, IQR, and the whiskers, 95% confidence intervals. Serum fluoride levels were significantly elevated in patients receiving voriconazole (median, 157 μg/L; IQR, 97 μg/L) compared with itraconazole (median < 30 μg/L; IQR, 28 μg/L), posaconazole (median < 30 μg/L, IQR, 0 μg/L), and the control group without antifungal medication (median < 30 μg/L; IQR, 28 μg/L). P < .001 for all comparisons.
Correlation between the glomerular filtration rate, as calculated by the CKD-EPI formula, and the serum fluoride levels for the patients taking voriconazole. Spearman rank correlation coefficient = 0.74; P < .001.
Correlation between the glomerular filtration rate, as calculated by the CKD-EPI formula, and the serum fluoride levels for the patients taking voriconazole. Spearman rank correlation coefficient = 0.74; P < .001.
Discussion
To the best of our knowledge, this is the first study to systematically analyze the impact of voriconazole on fluoride levels and skeletal disease in hematologic patients. Fluoride levels are elevated in all patients taking voriconazole irrespective of treatment duration. However, severe periostitis and skeletal disease are relatively rare and most probably result from prolonged therapy, impaired renal function, and consecutively persistent high fluoride serum levels.
The clinical presentation and the radiologic findings of the affected patients are impressive and can lead to considerable confusion as, for example, in our institution where the first affected patients have been mistakenly treated for chronic musculoskeletal GVHD.
Voriconazole-induced skeletal disease shares many features of skeletal fluorosis, a condition that has been known for decades.10-12 In Western countries, fluoride poisoning is rare but has been linked to occupational exposure, ingestion of tainted wine, excessive inhalation of fluorinated anesthetics, and iatrogenic because of the prescription of fluoride supplements.13-16 The World Health Organization states that a daily fluoride intake of > 6 mg bears a risk of skeletal events.17
Voriconazole contains 3 fluoride atoms, which account for 16.3% of the total molecular weight of the compound. It has an oral bioavailability of 96%. Thus, in theory, the calculated daily fluoride intake at a standard dose may be as high as 62.6 mg and therefore exceeds 10-fold the fluoride toxicity threshold defined by the World Health Organization.
But why do only some patients on voriconazole treatment develop clinically relevant skeletal disease? (1) Fluoride is mainly excreted by the kidneys, and an impaired renal function leads to higher circulating fluoride levels.18,19 Indeed, the fluoride level in our voriconazole treatment group was inversely correlated to the glomerular filtration rate, and all our patients with proven voriconazole-induced skeletal disease had moderate to severe chronic renal failure. However, chronic renal failure alone does not necessarily translate into skeletal disease as, for example, 6 additional patients with voriconazole treatment and moderate chronic renal failure did not show evidence of toxicity. (2) It is known from endemic fluorosis that prolonged intake promotes toxicity, and it seems reasonable to assume that this also holds true for patients exposed to fluorinated drugs, even though our data lack the statistical power to test this. (3) We hypothesize that pharmacogenetics and drug-drug interactions may account in part for the interindividual differences. (4) We speculate that inflammatory processes play an additional role as the symptoms in our patients were temporarily attenuated by adding corticosteroids and methotrexate and the pain rapidly resolved after cessation of voriconazole, even though the radiographically visible skeletal changes clearly needed more time to resolve.
Posaconazole, another triazole drug containing fluorinated moieties, did not cause fluoride excess in our patient cohort. However, the number of patients receiving posaconazole was rather small, the treatment duration short, and the median serum level low. Nevertheless, in 2 patients on long-term posaconazole treatment (≥ 20 days) and chronic renal failure (glomerular filtration rate < 90 mL/min), no fluoride excess was observed.
There are limitations of our study. First, it is a retrospective single-center study, which lacks the power to draw firm conclusions regarding the prevalence of the disorder and its observational, descriptive character does not allow statements about the precise mechanism of disease. Second, because of the retrospective nature of the radiologic assessment, no systematic radiographic screening was available. This might have led to a bias regarding the detection rate of skeletal disease. Third, more data for long-term posaconazole treatment must be obtained to draw definite conclusions on fluoride levels and possible associated clinical implications for patients receiving this drug.
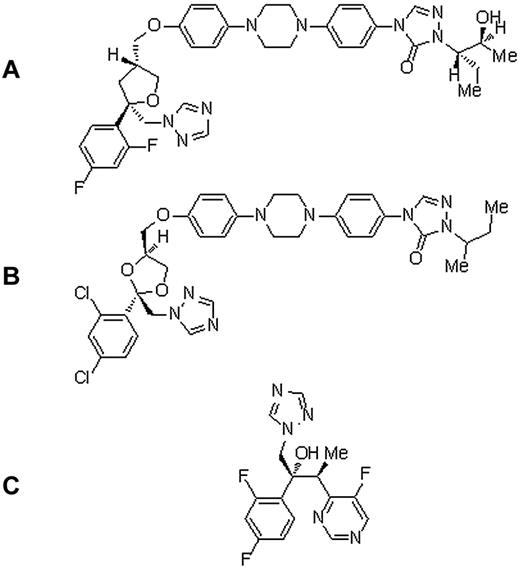
So far, no treatment of voriconazole-induced periostitis or chronic fluoride intoxication has been established, except withdrawal of the causing agent. Whereas antifungal treatment was stopped permanently in 2 of our patients, the third was put on itraconazole, another triazole drug yet containing chlorinated, but not fluorinated, moieties (Figure 5). Bone pain resolved rapidly in all patients.
Chemical structures. (A) Posaconazole. (B) Itraconazole. (C) Voriconazole.
In conclusion, voriconazole-induced periostitis and skeletal disease can occur in hematologic patients; and even though causality cannot yet be fully proven, fluoride is likely to contribute to the clinical picture. Physicians caring for patients on long-term voriconazole should be aware of this side effect, as it severely affects the quality of life and, when misdiagnosed, may lead to inappropriate therapy. In our experience, bone scintigraphy is a useful test when a voriconazole-induced periostitis is suspected, but other causes of skeletal disease still have to be ruled out. On discontinuation of voriconazole, skeletal disease seems to be fully reversible over time.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Pietro Butti for critical review of the manuscript and helpful comments.
Authorship
Contribution: B.G. and U.S. conceived and designed the study; B.G., R.G., D.F., and G.N. collected and assembled the data; B.G., M.G.M., G.S., and U.S. analyzed and interpreted the data; B.G. prepared the first draft of the manuscript; and all authors contributed to writing of the manuscript and gave final approval of the manuscript.
Conflict-of-interest disclosure: B.G., G.N., U.S. and M.G.M. received research grants from MSD Sharp & Dohme unrelated to this study. The remaining authors declare no competing financial interests.
Correspondence: Bernhard Gerber, Division of Hematology, University Hospital Zurich, Raemistrasse 100, CH-8091 Zurich, Switzerland; e-mail: bernhard.gerber@usz.ch.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal