Abstract
Allogeneic hematopoietic cell transplantation (HCT) is an effective treatment for adult T-cell leukemia (ATL), raising the question about the role of graft-versus-leukemia effect against ATL. In this study, we retrospectively analyzed the effects of acute and chronic graft-versus-host disease (GVHD) on overall survival, disease-associated mortality, and treatment-related mortality among 294 ATL patients who received allogeneic HCT and survived at least 30 days posttransplant with sustained engraftment. Multivariate analyses treating the occurrence of GVHD as a time-varying covariate demonstrated that the development of grade 1-2 acute GVHD was significantly associated with higher overall survival (hazard ratio [HR] for death, 0.65; P = .018) compared with the absence of acute GVHD. Occurrence of either grade 1-2 or grade 3-4 acute GVHD was associated with lower disease-associated mortality compared with the absence of acute GVHD, whereas grade 3-4 acute GVHD was associated with a higher risk for treatment-related mortality (HR, 3.50; P < .001). The development of extensive chronic GVHD was associated with higher treatment-related mortality (HR, 2.75; P = .006) compared with the absence of chronic GVHD. Collectively, these results indicate that the development of mild-to-moderate acute GVHD confers a lower risk of disease progression and a beneficial influence on survival of allografted patients with ATL.
Introduction
Adult T-cell leukemia (ATL) is a mature T-cell neoplasm that is causally associated with a retrovirus designated human T-cell leukemia virus type I (HTLV-I).1–4 HTLV-I is endemic in southwestern Japan, sub-Saharan Africa, the Caribbean Basin, and South America.3,4 In Japan, more than 1 million people were estimated to be infected with HTLV-I. Although the majority of HTLV-I–infected individuals remain asymptomatic throughout their lives, ∼ 5% develop ATL at a median age of 40 to 60 years.4,5
ATL is categorized into 4 clinical variants according to its clinical features: smoldering, chronic, acute, and lymphoma types.6 The acute and lymphoma variants of ATL have an extremely poor prognosis, mainly because of resistance to a variety of cytotoxic agents and susceptibility to opportunistic infections; the median survival time is ∼ 13 months with conventional chemotherapy,7,8 although encouraging results have been recently reported with the use of novel agents such as mogamulizumab.9–11
Over the past decade, allogeneic hematopoietic cell transplantation (HCT) has been increasingly performed with the aim of improving dismal prognosis of patients who developed ATL.12–18 Notably, some patients with ATL who relapsed after allogeneic HCT were shown to achieve remission only with the cessation of immunosuppressive agents, raising the question of whether the graft-versus-leukemia effect against ATL can be induced as part of graft-versus-host reaction.19,20 In 1 study, among 10 patients who experienced relapse of ATL after transplantation and were withdrawn from immunosuppressive therapy, 8 developed graft-versus-host disease (GVHD), and 6 of them subsequently achieved complete remission of ATL.19 Similar observations have been rarely reported in other aggressive mature lymphoid neoplasms,21 suggesting the unique susceptibility of ATL to graft-versus-host reactions. Recently, a combined analysis of 2 prospective studies including 29 ATL patients in total undergoing allogeneic HCT suggested that development of mild acute GVHD favorably affected overall survival and progression-free survival.22 However, the impact of GVHD on the outcome of allogeneic HCT in ATL needs to be verified in a much larger cohort. We previously conducted a nationwide retrospective study to evaluate the current results of allogeneic HCT for ATL, and we confirmed that a substantial proportion of patients with ATL can enjoy long-term, disease-free survival after transplantation: the overall survival rate at 3 years among patients who received transplants in complete remission and not in complete remission was 51% and 26%, respectively.23 Using the same cohort, we further evaluated the effects of acute and chronic GVHD on long-term outcomes of allografted patients with ATL.
Methods
Collection of data
Data on 417 patients with acute or lymphoma type ATL who had undergone allogeneic bone marrow, peripheral blood, or cord blood transplantation between January 1, 1996, and December 31, 2005, were collected through the Japan Society for Hematopoietic Cell Transplantation (JSHCT), the Japan Marrow Donor Program (JMDP), and the Japan Cord Blood Bank Network (JCBBN), the 3 largest HCT registries in our country; their roles were detailed previously.23 The patients were included from 102 transplant centers; the data were updated as of December 2008. The study was approved by the data management committees of JSHCT, JMDP, and JCBBN, as well as by the institutional review boards of Kyoto University Graduate School of Medicine, where this study was organized.
Inclusion and exclusion criteria
Patients were included in the analysis if the following data were available: age at transplantation, sex of the recipient, donor type, stem cell source, agents used in the conditioning regimen and GVHD prophylaxis, the maximum grade and day of occurrence of acute GVHD, and the day of neutrophil recovery. Acute GVHD was reported according to the traditional criteria,24 except that 1 patient was considered to have late-onset acute GVHD at day 133; neutrophil recovery was considered to have occurred when an absolute neutrophil count exceeded 0.5 × 109/L for 3 consecutive days after transplantation. Patients who missed any of these data (n = 37), who had a history of prior autologous or allogeneic HCT (n = 8), who had received an ex vivo T cell–depleted graft (n = 1), who experienced primary or secondary graft failure (n = 24) were excluded from the analysis. Because the association between the occurrence of acute GVHD and disease-associated mortality was difficult to evaluate in the event of early toxic death, patients who died within 30 days of transplantation (n = 53) also were excluded from the study. Among these 53 patients, 22 were evaluable for acute GVHD: grade 0 in 17 patients, grade 1-2 in 3 patients, and grade 3-4 in 2 patients. Two physicians (J.K. and T.I.) independently reviewed the quality of collected data, and 294 patients in total (158 males and 136 females), with a median age of 51 years (range, 18-79 years), were found to meet these criteria and included in the study: 163 patients from JSHCT, 82 patients from JMDP, and 49 patients from JCBBN. No overlapping cases were identified. Of these 294 patients, the effects of chronic GVHD, reported and graded according to using traditional criteria,25 were considered evaluable for the 183 patients who survived at least 100 days after transplantation with complete information on the type and the day of occurrence of chronic GVHD.
End points
The primary end point of the study was the effect of acute GVHD on overall survival, defined as the period from the date of transplantation until the date of death from any cause or the last follow-up. The secondary end points of the study included the impact of acute GVHD on disease-associated and treatment-related mortality, and the impact of chronic GVHD on overall survival, disease-associated mortality, and treatment-related mortality. Reported causes of death were reviewed and categorized into disease-associated or treatment-associated deaths. Disease-associated deaths were defined as deaths from relapse or progression of ATL, whereas treatment-related deaths were defined as any death other than disease-associated deaths.
Statistical analysis
The probability of overall survival was estimated by the Kaplan-Meier method. Treatment-related and disease-associated mortality were estimated with the use of cumulative incidence curves to accommodate the following competing events26 : disease-associated death for treatment-related mortality and treatment-related deaths for disease-associated mortality. Data on patients who were alive at the time of last follow-up were censored. Semi-landmark plots were used to illustrate the effects of GVHD on overall survival and cumulative incidence of disease-associated and treatment-related deaths. For patients with acute or chronic GVHD, the probability of overall survival and the cumulative incidences of disease-associated and treatment-related deaths were plotted as a function of time from the onset of acute or chronic GVHD. Day 24.5, the median day of onset for acute GVHD, was termed as the landmark day in patients without acute GVHD. In the case of patients without chronic GVHD, day 116, the median day of onset for chronic GVHD, was termed as the landmark day.
Univariate and multivariate Cox proportional hazards regression models were used to evaluate variables potentially affecting overall survival, whereas the Fine and Gray proportional subdistribution hazards models were used to evaluate variables potentially affecting disease-associated and treatment-related mortality.27 In these regression models, the occurrence of acute and chronic GVHD was treated as a time-varying covariate.28 In the analysis of acute GVHD, patients were assigned to the “no acute GVHD group” at the time of transplantation and then transferred to the “grade 1-2 acute GVHD group” or to the “grade 3-4 acute GVHD group” at the onset of the maximum grade of acute GVHD. In the analysis of chronic GVHD, patients were assigned to the “no chronic GVHD group” at the time of transplantation and then transferred to the “limited chronic GVHD group” or to the “extensive chronic GVHD group” at the onset of the maximum grade of chronic GVHD. The variables considered were the age group of the recipient (≤ 50 years or > 50 years at transplantation), sex of the recipient (female or male), disease status before transplantation (complete remission, disease status other than complete remission, or unknown), intensity of conditioning regimen (myeloablative, reduced intensity, or unclassifiable), type of GVHD prophylaxis (cyclosporine-based, tacrolimus-based, or other), type of donor (HLA-matched related donor, HLA-mismatched related donor, unrelated donor for bone marrow, or unrelated cord blood), time from diagnosis to transplantation (within 6 months, > 6 months, or unknown), and year of transplantation (1995-2002 or 2003-2005). We classified the intensity of conditioning regimen as myeloablative or reduced intensity based on the working definition by Center for International Blood and Marrow Transplant Research if data on dosage of agents and total-body irradiation (TBI) used in the conditioning regimen were available.29 For 110 patients for whom such information was not fully available, we used the information on conditioning intensity (myeloablative or reduced intensity) reported by treating clinicians. The cutoff points for year of transplantation were chosen such that we could make optimal use of the data with a proviso that the smaller group contained at least 30% of patients. In the analysis of the effect of chronic GVHD, the prior history of grade 2-4 acute GVHD also was added to the multivariate models. We also assessed the interaction between acute GVHD and the intensity of conditioning regimen in the multivariate models. Only factors with a P value of less than .10 in univariate analysis were included in the multivariate models. In addition, the heterogeneities of the effects of grade 1-2 or grade 3-4 acute GVHD on overall survival according to background transplant characteristics were evaluated by the forest plots stratified by variables included in the regression analyses. Furthermore, landmark analysis treating the development of acute GVHD as a time-fixed covariate was performed to confirm the results of analyses treating the occurrence of acute GVHD as a time-varying covariate; the landmark day was set at day 68 after transplantation, the date until when more than 95% of patients developed acute GVHD.
Results are expressed as hazard ratios (HRs) and their 95% confidence intervals (CI). All tests were 2-sided, and a P value of less than .05 was considered to indicate statistical significance. All statistical analyses were performed with STATA Version 11 software (StataCorp).
Results
Characteristics of patients and transplants
Characteristics of the patients and transplants are shown in Table 1. Most of the patients received transplants at the age of 41 to 60 years (median, 51 years). The disease status at transplantation was mainly defined as other than complete remission. The intensity of conditioning regimen was classified as myeloablative in 102 (35%) patients and reduced intensity in 128 (44%) patients; the remaining 64 (22%) patients were reported to receive cyclophosphamide plus TBI in 16 patients; busulfan plus cyclophosphamide in 15 patients; busulfan plus melphalan in 1 patient; purine analog-containing regimen in 6 patients; and other TBI-based regimens in 26 patients, although the intensity of these regimens was considered unclassifiable because of lack of dosage information. Cyclosporine-based prophylaxis against GVHD was used in more than half of patients. Patients underwent transplantation using HLA-matched related donor in 132 patients (45%), HLA-mismatched related donor in 31 patients (11%), unrelated bone marrow donor in 82 patients (28%), and unrelated cord blood unit in 49 patients (17%). Half of the patients received transplants within 6 months of diagnosis. The median time of follow-up among the survivors was 42.8 months (range, 1.5-102.3 months).
Characteristics of patients and transplants
| Variable . | No. of patients, n = 294 (%) . |
|---|---|
| Age group at transplant, y | |
| ≤ 30 | 7 (2) |
| > 30-40 | 30 (10) |
| > 40-50 | 109 (37) |
| > 50-60 | 123 (42) |
| > 60 | 25 (9) |
| Sex | |
| Male | 158 (54) |
| Female | 136 (46) |
| Disease status | |
| Complete remission | 99 (34) |
| Not in complete remission | 178 (61) |
| Unknown | 17 (6) |
| Conditioning regimen | |
| Myeloablative | 102 (34) |
| Reduced intensity | 128 (44) |
| Unclassifiable | 64 (22) |
| GVHD prophylaxis* | |
| Cyclosporine-based | 195 (66) |
| Tacrolimus-based | 94 (32) |
| Other | 5 (2) |
| Source of stem cells | |
| Bone marrow | 132 (45) |
| Peripheral blood | 111 (38) |
| Bone marrow + peripheral blood | 2 (1) |
| Cord blood | 49 (17) |
| Type of donor† | |
| HLA-matched related | 132 (45) |
| HLA-mismatched related | 31 (11) |
| Unrelated, bone marrow | 82 (28) |
| Unrelated, cord blood | 49 (17) |
| Time from diagnosis to transplant | |
| ≤ 6 mo | 141 (48) |
| > 6 mo | 141 (48) |
| Uncertain/missing | 12 (4) |
| Year of transplant | |
| 1995-1999 | 22 (7) |
| 2000-2002 | 91 (31) |
| 2003-2005 | 181 (62) |
| Follow-up of survivors | |
| Median time, mo (range) | 42.8 (1.5-102.3) |
| Variable . | No. of patients, n = 294 (%) . |
|---|---|
| Age group at transplant, y | |
| ≤ 30 | 7 (2) |
| > 30-40 | 30 (10) |
| > 40-50 | 109 (37) |
| > 50-60 | 123 (42) |
| > 60 | 25 (9) |
| Sex | |
| Male | 158 (54) |
| Female | 136 (46) |
| Disease status | |
| Complete remission | 99 (34) |
| Not in complete remission | 178 (61) |
| Unknown | 17 (6) |
| Conditioning regimen | |
| Myeloablative | 102 (34) |
| Reduced intensity | 128 (44) |
| Unclassifiable | 64 (22) |
| GVHD prophylaxis* | |
| Cyclosporine-based | 195 (66) |
| Tacrolimus-based | 94 (32) |
| Other | 5 (2) |
| Source of stem cells | |
| Bone marrow | 132 (45) |
| Peripheral blood | 111 (38) |
| Bone marrow + peripheral blood | 2 (1) |
| Cord blood | 49 (17) |
| Type of donor† | |
| HLA-matched related | 132 (45) |
| HLA-mismatched related | 31 (11) |
| Unrelated, bone marrow | 82 (28) |
| Unrelated, cord blood | 49 (17) |
| Time from diagnosis to transplant | |
| ≤ 6 mo | 141 (48) |
| > 6 mo | 141 (48) |
| Uncertain/missing | 12 (4) |
| Year of transplant | |
| 1995-1999 | 22 (7) |
| 2000-2002 | 91 (31) |
| 2003-2005 | 181 (62) |
| Follow-up of survivors | |
| Median time, mo (range) | 42.8 (1.5-102.3) |
Data are numbers (%) unless specified otherwise.
Cyclosporine-based indicates cyclosporine with or without other agents; tacrolimus-based indicates tacrolimus with or without other agents.
HLA compatibility was defined according to the results of serologic or low-resolution molecular typing for HLA-A, B, and DR antigens.
Effects of acute GVHD on overall survival
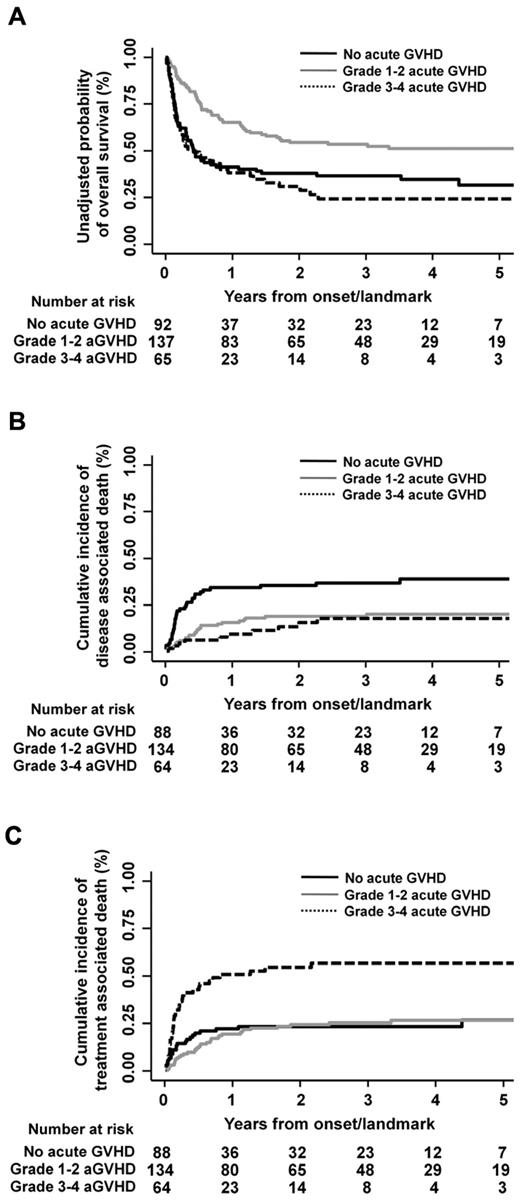
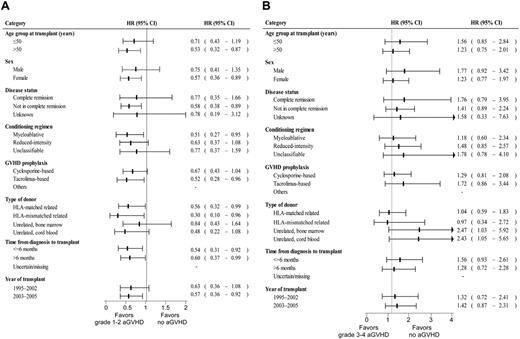
The median onset day of acute GVHD of any grade after transplantation was 24.5 (range, 5-133). Acute GVHD of grades 1-4, 2-4, and 3-4 occurred in 202 patients (69%), 150 patients (51%), and 65 patients (22%), respectively. The effect of acute GVHD on overall survival was evaluated using semi-landmark plots with reference to the following 3 categories: no acute GVHD, grade 1-2 acute GVHD, and grade 3-4 acute GVHD (Figure 1A). The impact of grade 1-2 or grade 3-4 acute GVHD on overall survival also was evaluated by forest plots stratified by background characteristics of patients and transplants (Figure 2). These analyses revealed that development of grade 1-2 acute GVHD was consistently associated with higher overall survival compared with the absence of acute GVHD, whereas occurrence of grade 3-4 acute GVHD was consistently associated with lower overall survival, except that adverse impact of grade 3-4 acute GVHD was not observed in the subgroups of patients who received transplants from an HLA-matched related or HLA-mismatched related donor. Multivariate analysis treating an occurrence of acute GVHD as a time-dependent covariate also confirmed the positive impact of grade 1-2 acute GVHD (HR, 0.65; 95% CI, 0.45-0.93; P = .018) and the adverse impact of grade 3-4 acute GVHD on overall survival (HR, 1.64; 95% CI, 1.10-2.42; P = .014; Table 2). Patients who received reduced intensity conditioning and myeloablative conditioning had similar rates of overall survival by both univariate (HR of reduced intensity vs myeloablative transplant, 1.19; 95% CI, 0.85-1.68; P = .318) and multivariate analysis (HR, 0.95; 95% CI, 0.61-1.47; P = .814). There was no interaction effect between conditioning intensity and grade 1-2 (P = .704) or grade 3-4 acute GVHD (P = .891) on overall survival. The effect of each grade of acute GVHD on overall survival was additionally evaluated. It showed that only grade 2 acute GVHD was associated with superior overall survival, whereas only grade 4 acute GVHD was associated with inferior survival (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In the landmark analysis treating an occurrence of acute GVHD as a time-fix covariate, consistent results were obtained for patients who survived at least 68 days (landmark day), although the adverse impact of grade 3-4 acute GVHD on overall survival became no longer significant (supplemental Table 2).
Semi-landmark plots for effects of acute GVHD. Semi-landmark plots illustrating the effects of acute GVHD on overall survival (A), disease-associated mortality (B), and treatment-related mortality (C).
Semi-landmark plots for effects of acute GVHD. Semi-landmark plots illustrating the effects of acute GVHD on overall survival (A), disease-associated mortality (B), and treatment-related mortality (C).
Impact of the grade of acute GVHD on overall survival in each stratified category. Effects of grade 1-2 (A) and grade 3-4 acute GVHD (B) on overall survival are shown as forest plots. Square boxes on lines indicate hazard ratios compared with “no acute GVHD group,” and horizontal lines represent the corresponding 95% CI. Abbreviations used are the same as described in the footnotes to Tables 1 and 2.
Impact of the grade of acute GVHD on overall survival in each stratified category. Effects of grade 1-2 (A) and grade 3-4 acute GVHD (B) on overall survival are shown as forest plots. Square boxes on lines indicate hazard ratios compared with “no acute GVHD group,” and horizontal lines represent the corresponding 95% CI. Abbreviations used are the same as described in the footnotes to Tables 1 and 2.
Effect of acute GVHD on overall survival, disease-associated mortality, and treatment-related mortality after allogeneic hematopoietic cell transplantation for adult T-cell leukemia
| Outcome . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | |
| Overall survival* | ||||
| Grade 1 or 2 acute GVHD vs no acute GVHD | 0.60 (0.42-0.85) | .004 | 0.65 (0.45-0.93) | .018 |
| Grade 3 or 4 acute GVHD vs no acute GVHD | 1.38 (0.94-2.01) | .099 | 1.64 (1.10-2.42) | .014 |
| Disease-associated mortality† | ||||
| Grade 1 or 2 acute GVHD vs no acute GVHD | 0.47 (0.28-0.79) | .005 | 0.54 (0.32-0.92) | .023 |
| Grade 3 or 4 acute GVHD vs no acute GVHD | 0.41 (0.21-0.81) | .010 | 0.44 (0.22-0.90) | .024 |
| Treatment-related mortality‡ | ||||
| Grade 1 or 2 acute GVHD vs no acute GVHD | 1.13 (0.67-1.89) | .649 | 1.22 (0.72-2.07) | .461 |
| Grade 3 or 4 acute GVHD vs no acute GVHD | 3.34 (1.94-5.74) | < .001 | 3.50 (2.01-6.11) | < .001 |
| Outcome . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | |
| Overall survival* | ||||
| Grade 1 or 2 acute GVHD vs no acute GVHD | 0.60 (0.42-0.85) | .004 | 0.65 (0.45-0.93) | .018 |
| Grade 3 or 4 acute GVHD vs no acute GVHD | 1.38 (0.94-2.01) | .099 | 1.64 (1.10-2.42) | .014 |
| Disease-associated mortality† | ||||
| Grade 1 or 2 acute GVHD vs no acute GVHD | 0.47 (0.28-0.79) | .005 | 0.54 (0.32-0.92) | .023 |
| Grade 3 or 4 acute GVHD vs no acute GVHD | 0.41 (0.21-0.81) | .010 | 0.44 (0.22-0.90) | .024 |
| Treatment-related mortality‡ | ||||
| Grade 1 or 2 acute GVHD vs no acute GVHD | 1.13 (0.67-1.89) | .649 | 1.22 (0.72-2.07) | .461 |
| Grade 3 or 4 acute GVHD vs no acute GVHD | 3.34 (1.94-5.74) | < .001 | 3.50 (2.01-6.11) | < .001 |
Other significant variables were sex of recipient, female (reference, 1.00) and male (HR, 1.70; 95% CI, 1.24-2.32; P = .001); achievement of complete remission, complete remission (reference, 1.00), status other than complete remission (HR, 2.05; 95% CI, 1.44-2.92; P < .001), and status not known (HR, 2.21; 95% CI, 1.15-4.22; P = .017); type of donor, HLA-matched related donor (reference, 1.00), HLA-mismatched related donor (HR, 1.71; 95% CI, 1.04-2.84; P = .036), unrelated donor of bone marrow (HR, 1.39; 95% CI, 0.94-2.06; P = .096), and unrelated cord blood (HR, 1.86; 95% CI, 1.22-2.83; P = .004).
Other significant variables were achievement of complete remission, complete remission (reference, 1.00), status other than complete remission (HR, 2.98; 95% CI, 1.62-5.47; P < .001), and status not known (HR, 0.96; 95% CI, 0.21-4.49; P = .963); type of donor, HLA-matched related donor (reference, 1.00), HLA-mismatched related donor (HR, 2.14; 95% CI, 1.00-4.55; P = .049), unrelated donor of bone marrow (HR, 1.45; 95% CI, 0.81-2.61; P = .214), and unrelated cord blood (HR, 1.25; 95% CI, 0.63-2.49; P = .517).
Another significant variable was achievement of complete remission, complete remission (reference, 1.00), status other than complete remission (HR, 1.17; 95% CI, 0.74-1.84; P = .498) and status not known (HR, 2.31; 95% CI, 1.04-5.15; P = .040).
Effects of acute GVHD on disease-associated and treatment-related mortality
We next evaluated the effects of acute GVHD on disease-associated and treatment-related mortality (Figure 1B-C). Disease-associated mortality was defined as cumulative incidence of death directly attributable to relapse or progression of ATL, whereas treatment-related mortality was calculated as cumulative incidence of any death not included in disease-associated deaths. Multivariate analysis revealed that disease-associated mortality was lower in the presence of grade 1-2 and grade 3-4 acute GVHD compared with the absence of acute GVHD (grade 1-2 acute GVHD: HR, 0.54; 95% CI, 0.32-0.92; P = .023 and grade 3-4 acute GVHD: HR, 0.44; 95% CI, 0.22-0.90; P = .024; Table 2), and each grade of acute GVHD showed consistent inverse association with disease-associated mortality (supplemental Table 1). Although the risk of treatment-related mortality was not higher in the presence of grade 1-2 acute GVHD, development of grade 3-4 acute GVHD was significantly associated with higher treatment-related mortality compared with the absence of acute GVHD (HR, 3.50; 95% CI, 2.01-6.11; P < .001; Table 2). Patients undergoing reduced intensity transplantation and those undergoing myeloablative transplantation had similar risks of disease-associated death (HR, 0.99; 95% CI, 0.46-2.13; P = .975) and treatment-related death (HR, 0.98; 95% CI, 0.60-1.59; P = .928) by multivariate analysis. There was no interaction effect between conditioning intensity and grade 1-2 or grade 3-4 acute GVHD on disease-associated mortality and treatment-related mortality. Of 95 patients who experienced treatment-related deaths, 27 patients succumbed to infectious complications: bacterial in 13 patients, viral in 7 patients (including 3 cases of cytomegalovirus disease), viral and bacterial in 1 patient, fungal in 5 patients, and no specific organism reported in 1 patient. The proportions of patients who died of infectious complication among those without acute GVHD (n = 92), those with grade 1-2 (n = 137), and those with grade 3-4 acute GVHD (n = 65) were 4%, 9%, and 17%, respectively (supplemental Table 3). By multivariate analysis, development of grade 3-4 acute GVHD was significantly associated with higher risk of death related to infection (HR, 4.74; 95% CI, 1.51-14.8; P = .008), whereas the adverse influence on the infection-related deaths was less evident in the presence of grade 1-2 acute GVHD (HR, 2.17; 95% CI, 0.72-6.56; P = .169).
Effects of chronic GVHD on overall survival and mortality
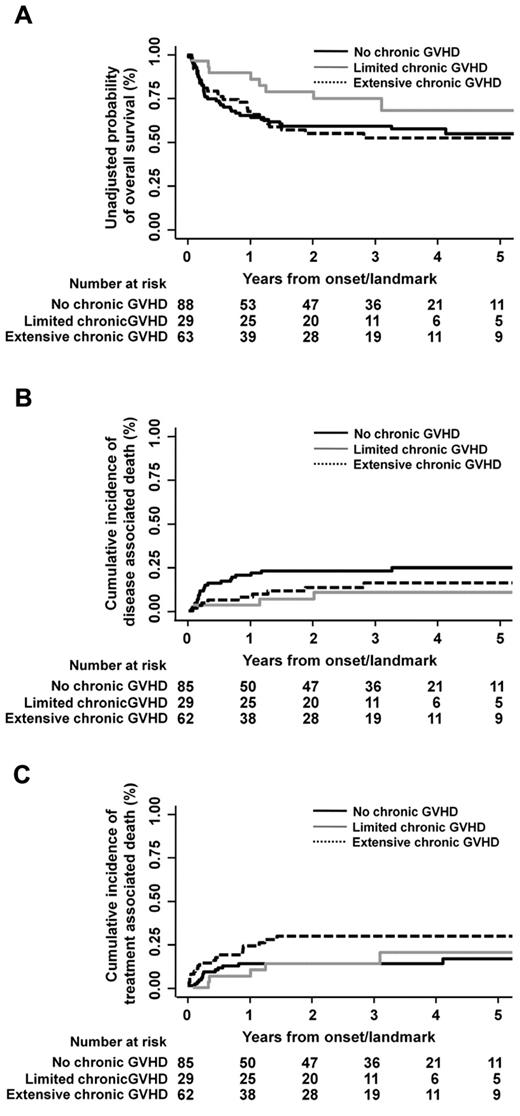
Chronic GVHD was evaluated in 183 patients who survived at least 100 days after transplantation. The median day of chronic GVHD occurrence after transplantation was 116 (range, 100-146 days). Limited and extensive chronic GVHD occurred in 29 (16%) and 63 patients (34%), respectively. Semi-landmark plots were constructed to illustrate the effects of chronic GVHD on overall survival, disease-associated mortality, and treatment-related mortality with reference to the following subgroups: no chronic GVHD, limited chronic GVHD, and extensive chronic GVHD (Figure 3). In multivariate analysis treating an occurrence of chronic GVHD as a time-dependent covariate, neither overall survival nor disease-associated mortality was significantly associated with severity of chronic GVHD, whereas treatment-related mortality was higher in the presence of extensive chronic GVHD (HR, 2.75; 95% CI, 1.34-5.63; P = .006) compared with the absence of chronic GVHD (Table 3). The proportions of patients who died of infectious complication among those without chronic GVHD (n = 91), those with limited chronic GVHD (n = 29), and those with extensive chronic GVHD (n = 63) were 7%, 10%, and 8%, respectively. In multivariate analysis, no statistically significant association was found between infection-related death and the occurrence of either limited (P = .289) or extensive GVHD (P = .836).
Semi-landmark plots for impact of chronic GVHD. Semi-landmark plots illustrating impact of chronic GVHD on overall survival (A), disease-associated mortality (B), and treatment-related mortality (C).
Semi-landmark plots for impact of chronic GVHD. Semi-landmark plots illustrating impact of chronic GVHD on overall survival (A), disease-associated mortality (B), and treatment-related mortality (C).
Effect of chronic GVHD on overall survival, disease-associated mortality, and treatment-related mortality after allogeneic hematopoietic cell transplantation for adult T-cell leukemia
| Outcome . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | |
| Overall survival* | ||||
| Limited chronic GVHD vs no chronic GVHD | 0.71 (0.34-1.47) | .353 | 0.72 (0.35-1.50) | .385 |
| Extensive chronic GVHD vs no chronic GVHD | 1.45 (0.90-2.35) | .131 | 1.40 (0.86-2.30) | .176 |
| Disease-associated mortality† | ||||
| Limited chronic GVHD vs no chronic GVHD | 0.45 (0.14-1.46) | .183 | 0.45 (0.14-1.44) | .178 |
| Extensive chronic GVHD vs no chronic GVHD | 0.81 (0.39-1.67) | .563 | 0.80 (0.39-1.64) | .536 |
| Treatment-related mortality‡ | ||||
| Limited chronic GVHD vs no chronic GVHD | 1.59 (0.64-3.95) | .316 | 1.56 (0.63-3.87) | .342 |
| Extensive chronic GVHD vs no chronic GVHD | 2.85 (1.41-5.77) | .004 | 2.75 (1.34-5.63) | .006 |
| Outcome . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | |
| Overall survival* | ||||
| Limited chronic GVHD vs no chronic GVHD | 0.71 (0.34-1.47) | .353 | 0.72 (0.35-1.50) | .385 |
| Extensive chronic GVHD vs no chronic GVHD | 1.45 (0.90-2.35) | .131 | 1.40 (0.86-2.30) | .176 |
| Disease-associated mortality† | ||||
| Limited chronic GVHD vs no chronic GVHD | 0.45 (0.14-1.46) | .183 | 0.45 (0.14-1.44) | .178 |
| Extensive chronic GVHD vs no chronic GVHD | 0.81 (0.39-1.67) | .563 | 0.80 (0.39-1.64) | .536 |
| Treatment-related mortality‡ | ||||
| Limited chronic GVHD vs no chronic GVHD | 1.59 (0.64-3.95) | .316 | 1.56 (0.63-3.87) | .342 |
| Extensive chronic GVHD vs no chronic GVHD | 2.85 (1.41-5.77) | .004 | 2.75 (1.34-5.63) | .006 |
There was no significant variable.
There was no significant variable.
There was no other significant variable.
Discussion
To our knowledge, this is the largest retrospective study to analyze the impact of acute and chronic GVHD on clinical outcomes including overall survival, disease-associated mortality, and treatment-related mortality after allogeneic HCT for ATL. In the present study, the occurrence of both grade 1-2 and grade 3-4 acute GVHD was associated with lower disease-associated mortality compared with the absence of acute GVHD. However, positive effect of GVHD on reduced disease-associated mortality was counterbalanced by increased treatment-related mortality among patients who developed severe acute GVHD, and an overall beneficial effect on survival was observed only with the development of mild-to-moderate acute GVHD. In contrast to acute GVHD, no beneficial effect was observed in association with the development of chronic GVHD, although the point estimate of the HR comparing limited chronic GVHD versus the absence of chronic GVHD suggested the trend toward a reduced risk of disease-associated deaths in the limited chronic GVHD group.
Our present findings are in contrast to the previous reports showing the beneficial effects of chronic GVHD rather than acute GVHD on the prevention of disease recurrence after allogeneic HCT. It is less likely that the particular characteristics of chronic GVHD in patients with ATL biased the results, because the incidence rate and median onset day of chronic GVHD in our cohort were similar to those reported in previous studies evaluating the incidence of chronic GVHD among Japanese patients, most of whom had received allogeneic HCT for myeloid neoplasms or acute lymphoblastic leukemia.30–32 Conceivably, the rapid tempo of disease recurrence of ATL might be such that chronic GVHD is less potent in terms of harnessing clinically relevant graft-versus-leukemia responses compared with acute GVHD. However, the results of our analysis regarding the effect of chronic GVHD should be interpreted with caution because the number of patients evaluable for chronic GVHD was relatively small in our study for providing sufficient statistical power. The effect of chronic GVHD on outcomes after HCT for ATL should be further explored in a larger cohort.
The occurrence of GVHD has been shown to exert a potent graft-versus-leukemia effect in terms of reducing relapse incidence in acute leukemia or chronic myeloid leukemia.33,34 In contrast, multiple studies have documented a correlation between GVHD in its acute or chronic form and treatment-related mortality. In a study of patients undergoing HLA-identical sibling HCT for chronic myeloid leukemia, the overall beneficial effect on long-term survival was demonstrated only in a group of patients who developed grade 1 acute GVHD or limited chronic GVHD.33 In another study of HLA-identical sibling HCT for leukemia using cyclosporine and methotrexate as GVHD prophylaxis, a benefit of mild GVHD was only seen in high-risk patients but not in standard-risk patients. Therefore, the therapeutic window between decreased relapse incidence and increased transplant-related mortality in association with the development of GVHD has been considered to be very narrow.34
With regard to the effectiveness of allogeneic HCT for ATL, it is also of note here that posttransplant eradication of ATL cells can be achieved without the use of high-dose chemoradiotherapy: patients who received a transplant with reduced intensity conditioning had survival outcomes similar to those who received a transplant with myeloablative conditioning in our study. Intriguingly, several small cohort studies exhibited that abrupt discontinuation of immunosuppressive agents resulted in disappearance or reduction in the tumor burden in allografted patients with ATL. In some cases, remission of ATL was observed along with the development of GVHD.19,20,22 Taken together with the findings of this study, it is suggested that ATL is particularly susceptible to immune modulation following allogeneic HCT. To clarify the presence of such “graft-versus-ATL” effect, further investigations are needed to assess the efficacy of donor lymphocyte infusion or withdrawal of immunosuppressive agents on relapse after transplantation.
Of the HTLV-I gene products, Tax is a dominant target of HTLV-I–specific cytotoxic T lymphocytes. The vigorous Tax-specific cytotoxic T-cell responses were demonstrated in recipients who obtained complete remission after allogeneic HCT for ATL, suggesting that “graft-versus-HTLV-I” responses might contribute to the eradication of ATL cells.35,36 However, Tax is generally undetectable or present in very low levels in primary ATL cells.37,38 In addition, small amounts of HTLV-I provirus can be detected in peripheral blood of recipients who attained long-term remission of ATL, even after HCT from HTLV-I–negative donors.39,40 These findings suggest that “graft-versus-ATL” effect can be harnessed without complete elimination of HTLV-I. It is also important to note that allogeneic HCT is emerging as an effective treatment option for other mature T-cell neoplasms not related to HTLV-I, such as mycosis fungoides/Sézary syndrome and various types of aggressive peripheral T-cell lymphomas.41,42 These observations raised the possibility that the common targets for alloimmune responses might exist across a spectrum of malignant T-cell neoplasms, including ATL. The minor histocompatibility antigens or tumor-specific antigens can be other targets of alloimmune anti-ATL effect.43–45 Therefore, the elucidation of the mechanism underlying an immunologic eradication of primary ATL cells may lead to a new strategy for improving outcomes of allogeneic HCT not only for ATL but also for other intractable T-cell neoplasms.
This study has several limitations. First, acute GVHD might be intentionally induced for some patients considered at high risk of relapse by treating clinicians. Second, the information on the day when each grade of GVHD occurred was not available. Therefore, we treated the development of acute and chronic GVHD in their worst severity as a time-varying covariate. To validate the results, we also performed the landmark analysis and obtained consistent results. Third, the relatively small number of patients with chronic GVHD might mask or bias the effect of chronic GVHD on outcomes. Last, the effect of multiple testing should be taken into account for the interpretation of the secondary end points.
In conclusion, the development of acute GVHD was associated with lower disease-associated mortality after allogeneic HCT for ATL compared with the absence of acute GVHD. However, improved survival can be expected only among a group of patients who developed mild-to-moderate acute GVHD because those who developed severe acute GVHD were at high risk of treatment-related mortality. New strategies that enhance the allogeneic anti-ATL effect without exacerbating GVHD are required to improve the outcomes of patients undergoing allogeneic HCT for ATL.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are indebted to all the physicians and data managers at the centers who contributed valuable data on transplantation for adult T-cell leukemia to the JSHCT, the JMDP, and the JCBBN. They also thank all the members of the data management committees of JSHCT, JMDP, and JCBBN for their dedicated management of data.
This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (T.U.).
The views expressed in this report are those of authors and do not indicate the views of the JSHCT, JMDP, or JCBBN.
This work is in memory of T.U., who died during the preparation of this manuscript.
Authorship
Contribution: T.I. and T.U. designed the research and organized the project; M. Hishizawa, J.K., T.I., and T.U. reviewed and analyzed data and wrote the paper; J.K., T.I., and K.M. performed statistical analysis; Y.A., R.S., and H.S. collected data from JSHCT; T.K. and Y. Morishima collected data from JMDP; T.N.-I., and S. Kato collected data from JCBBN; and A.U., S.T., T.E., Y. Moriuchi, R.T., F.K., Y. Miyazaki, M.M., K.N., M. Hara, M.T., S. Kai, and J.O. interpreted data and reviewed and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A list of other members who contributed data on allogeneic HSCT for ATL to JSHCT, JMDP, and JCBBN appears in the online supplemental Appendix.
Correspondence: Tatsuo Ichinohe, Department of Hematology and Oncology, Graduate School of Medicine, Kyoto University, 54 Shogoin Kawaharacho, Sakyo-ku, Kyoto 606-8507, Japan; e-mail: nohe@kuhp.kyoto-u.ac.jp.
References
Author notes
J.K. and M.H. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal