Abstract
Role of interim-PET (I-PET) in diffuse large B-cell Lymphoma (DLBCL) is controversial. To determine predictive value of I-PET on progression-free survival (PFS), we enrolled 88 first-line DLBCL patients treated with 6-8 R-CHOP courses regardless of I-PET. PET/CT were performed at diagnosis, after 2 to 4 courses and at the end of therapy with central reviewing according to visual dichotomous criteria. Results are as follows: I-PET, 72% negative, 28% positive; final-PET (F-PET), 88% negative, 12% positive; clinical complete response 90%. Concordance between clinical response and F-PET negativity was 97% because of 2 false positive. With a median follow-up of 26.2 months, 2-year overall survival and PFS were 91% and 77%, respectively. Two-year PFS for I-PET and F-PET negative versus positive were as follows: I-PET 85% versus 72% (P = .0475); F-PET 83% versus 64% (P < .001). Because of a small number of events, 2 independent bivariate Cox models were tested for PFS. In model 1, F-PET contradicted I-PET (hazard ratio [HR] = 5.03, P = .015 vs 1.27, P = 691); in model 2, F-PET (HR = 4.54) and International propnostic Index score (HR = 5.36, P = .001) remained independent prognostic factors. In conclusion, positive I-PET is not predictive of a worse outcome in DLBCL; larger prospective studies and harmonization of I-PET reading criteria are needed.
Introduction
Despite attempts to increase the efficacy of conventional chemotherapy, no more than 60% of patients diagnosed with diffuse large B-cell lymphoma (DLBCL) are potentially cured with a prolonged progression-free survival (PFS), when treated with rituximab-CHOP-like regimens. The current salvage therapy seems to be inadequate in nonresponding patients: only 30% to 35% of resistant or relapsed patients can achieve a prolonged PFS with high-dose chemotherapy followed by autologous stem cell transplantation in the rituximab era.1 Indeed, a good strategy might be a first-line risk-tailored therapy in the poor prognosis patients.
The prognosis of DLBCL is mostly established on the basis of the clinical characteristics defined by the International Prognostic Index (IPI) and more recently by the gene expression profiling subtypes.2,3 IPI is a recognized predictor of treatment outcome, but there is a considerable variation between the outcome of individual patients within the same IPI prognostic group. Response to treatment may be another important predictor of outcome with the advantage of addressing the management for the individual patient. The monitoring of treatment efficacy is generally performed with evaluation of tumor size using conventional anatomic imaging modalities, commonly computed tomography (CT). Unfortunately, CT has the disadvantage of not distinguishing between a real tumor mass and residual tissue mass; in addition, assessment of early response is not reliable during treatment with CT because tumor volume reduction may require time.
In the diagnosis of DLBCL, 18-fluorodeoxyglucose positron emission tomography (18-FDG-PET/CT) has proved to be highly sensitive in determining sites of disease.4–6 In addition, residual FDG positivity at the end of therapy is predictive for survival.5–8 Initial reports suggested that PET/CT scans, performed early during treatment (interim-PET [I-PET]) after 2 to 4 courses of CHOP chemotherapy, could identify patients likely to relapse.9,10 Itti et al reported the value of I-PET in predicting the outcome of DLBCL patients with different histologic subtypes who had been treated with different chemotherapy regimens (R-CHOP, ACVBP) using a Delta-SUV-based criteria10,11 ; however, a significant portion of patients had a prolonged survival in a remission condition despite positive I-PET scan. Indeed, the significance of I-PET in aggressive non-Hodgkin lymphoma is still unconfirmed, and its use is not approved outside of clinical trials.6,11,12
In our multicenter retrospective study, we analyzed a homogeneous cohort of newly diagnosed DLBCL patients treated with R-CHOP who underwent PET scan at diagnosis, during and at the end of treatment. The aim of our study was to determine the predictive value of I-PET and final-PET (F-PET) on PFS by visual dichotomous analysis in DLBCL patients.
Methods
Patient population and treatment
Eighty-eight newly diagnosed DLBCL patients were included in this study. The study was approved by the San Giovanni Battista Hospital and University ethical committee. The patients were referred to 5 different hematology departments between April 2004 and October 2009. Baseline assessment included: bone marrow biopsy; full laboratory workup; HIV serology; chest, abdomen, and pelvis CT scans; multigated acquisition scan or echocardiography. In addition, they all had FDG-PET scans at staging, during therapy (I-PET), and at the end of therapy (F-PET). Final response was evaluated at least one month after the end of chemoimmunotherapy with or without radiotherapy. Complete remission (CR) and partial remission was defined according to the Cheson 2007 criteria.5 No response was defined as any response less than a partial remission, progressive disease, or death during the treatment period.
Follow-up data were recorded at scheduled visits. No patient was lost to follow-up. All patients were treated according to departmental protocol. Depending on the stage and site of presentations, patients were given either R-CHOP alone or a combination of R-CHOP and radiotherapy. All patients were treated with standard R-CHOP2113 or dose-dense R-CHOP1414 for 6 or 8 courses; therapy was performed as planned and never modified by I-PET results. Involved field radiotherapy was delivered to areas of bulky disease regardless of PET results. G-CSF was given if neutropenia grade 4 occurred during chemotherapy in R-CHOP21 patients according to primary physician policy and in all R-CHOP14 patients.
PET scans
All studies were 18F-FDG-PET/CT scans, acquired from the orbits to the proximal third of the thighs. Scans were acquired, respectively, on the following tomographs: Philips Gemini, General Electric Discovery ST, General Electric Discovery LS, and Siemens Biograph 16 HI-REZ. All patients fasted at least 6 hours before intravenous injection of 37 MBq/10 kg of 18F-glucose. All patients had glucose levels between 90 and 160 mg/dL at the moment of injection; all scans were done within a range of 60 to 90 minutes after injection. The PET scans were performed at 60 minutes in 82 of 88 patients; the remaining 6 patients had PET scans acquired later than usual (at 90 minutes) because of practical issues, such as delays in arrival of patients or materials, unexpected hyperglycemia, and incorrect transmission of clinical data, etc, leading to last-minute rescheduling of the daily work plan. However, if this happened at staging, the following studies were scheduled to respect the initial time point, so the near totality of patients had scans acquired at the same timing.
PET scans were performed in all patients at diagnosis, during treatment (I-PET) after 2, 3, or 4 cycles of chemotherapy, and at the end of therapy (F-PET) approximately 1 month after the end of chemoimmunotherapy with or without radiotherapy. Fifty-eight patients had I-PET performed after 2 courses, and 30 patients had I-PET performed after 3 (9 patients) or 4 (21 patients) courses of chemotherapy.
All interim results were interpreted as positive or negative by visual dichotomous response criteria according to the 5-point score system defined at the First Consensus Conference in Deauville 2009,15 defined as follows: 1 indicates no uptake; 2, uptake equal or less than mediastinum; 3, uptake more than mediastinum but less than liver; 4, uptake moderately increased compared with the liver at any site; and 5, uptake markedly increased compared with the liver at any site or/and new sites of disease.
We set the cut-off value at grade 4 of the 5-point score. When appreciation of difference on a visual basis was difficult, a SUVmax-based analysis (liver vs lesion) was performed.
The results of all scans were centrally reviewed at the Turin University Center. A δSUV analysis between baseline and I-PET (Basal-Interim δSUVmax) or between baseline and F-PET (Basal-Final δSUVmax) was also performed on a subgroup of 46 patients available and quantifiable directly at the Turin Center. The methodology was the same as that used by Lin et al16 : using a steep color scale and normalizing at the liver, δSUVmax between basal and interim scan was referred to the hottest lesion at each study, with a cut-off value at 66%.
Statistical methods
Demographic and baseline disease characteristics were recorded, censoring the times of observation on June 30, 2010, at a median follow-up of 26.2 months (range, 8-67 months). The primary endpoint was PFS, and the secondary end point was overall survival (OS), according to the Cheson et al criteria.5 PFS was defined as the time from the start of treatment to death/progression as a result of any cause; patients still alive were censored at the date of last contact. OS was defined as the time from the start of treatment to death as a result of any cause; patients still alive were censored at the date of last contact.
Patient characteristics were compared using the Fisher exact test for discrete variables and the Mann-Whitney test for continuous ones.
PFS and OS were analyzed by the Cox proportional hazards model, comparing the 2 arms by the Wald test and calculating 95% CIs. The univariate analyses were performed for the following variables: sex (female vs male), age at diagnosis (> 60 vs ≤ 60 years), Ann Arbor stage (III or IV vs I or II), ECOG performance status (≥ 2 vs 0 or 1), lactate dehydrogenase (LDH; abnormal vs ≤ normal), extranodal involvement (≥ 2 vs 0 or 1), IPI score (≥ 3 vs 0-2), bulky disease/bone marrow/spleen involvement (any vs none), G-CSF/radiotherapy administration (any vs none), responses at I-PET and F-PET (positive vs negative), number of R-CHOP cycles at I-PET (2 vs 3 or 4), R-CHOP interval (21 vs 14 days), and clinical response at the end of the first-line chemotherapy (no CR vs CR). Because of the small number of events (20 progressions in the whole population), the Cox proportional hazards model was then used in 2 independent bivariate analyses to assess the effect of different prognostic factors on PFS and the possible confounding role of the response at F-PET.
All P values were obtained by 2-sided exact method at the conventional 5% significance level. Data were analyzed as of December 2010 by SPSS Version 18.0.1.
Results
Clinical characteristics
The study included 88 patients. Clinical features were: median age 55 years (range, 18-80 years); 41 males and 47 females; 29 patients were in stages I or II and 59 in stages III or IV, respectively; a low/low intermediate International Prognostic Index score (IPI 0-2) was defined in 53 patients and an intermediate/intermediate high/high IPI score (IPI 3-5) in 35. Twenty-seven patients had bulky disease, and LDH was higher than normal in 42. Twenty patients had bone marrow involvement, 65 patients had 0 or 1 extranodal involvement, and 23 had more than 2 extranodal sites.
All patients underwent initial staging 18F-FDG-PET/CT. I-PET was performed after 2 cycles of R-chemotherapy in 58, or 3 or 4 cycles in 30 patients. The median time to perform I-PET from the previous course was 13 days (range, 4-27 days). F-PET scans were performed 36 days (range, 12-210 days) after the end of R-chemotherapy. Thirty-one patients received R-CHOP21, and 57 received R-CHOP14. Involved field radiotherapy to areas of bulky disease was delivered to 14 patients regardless of PET results. G-CSF was given to 21 of 31 R-CHOP21 patients and in all 57 R-CHOP14 patients. Clinical characteristics are summarized in Table 1.
Clinical characteristics
| Characteristic . | All patients (n = 88), no. (%) of patients . | I-PET negative (n = 63), no. (%) of patients . | I-PET positive (n = 25), no. (%) of patients . | P . |
|---|---|---|---|---|
| Age | ||||
| > 60 y | 35 (40) | 28 (44) | 7 (28) | .227 |
| < 60 y | 53 (60) | 35 (56) | 18 (72) | |
| Sex | ||||
| Female | 47 (53) | 29 (46) | 18 (72) | .034 |
| Male | 41 (47) | 34 (54) | 7 (28) | |
| Ann Arbor stage | ||||
| III or IV | 59 (67) | 37 (59) | 22 (88) | .011 |
| I or II | 29 (33) | 26 (41) | 3 (12) | |
| ECOG Performance Status | ||||
| > 2 | 25 (28) | 15 (24) | 10 (40) | .189 |
| 0 to 1 | 63 (72) | 48 (76) | 15 (60) | |
| LDH | ||||
| > 450 U/L | 42 (48) | 28 (44) | 14 (56) | .354 |
| < 450 U/L | 46 (52) | 35 (56) | 11 (44) | |
| Extranodal involvement | ||||
| > 2 | 23 (26) | 13 (21) | 10 (40) | .104 |
| 0 or 1 | 65 (74) | 50 (79) | 15 (60) | |
| Age-adjusted IPI risk | ||||
| > 3 | 35 (40) | 23 (37) | 12 (48) | .344 |
| 0-2 | 53 (60) | 40 (63) | 13 (52) | |
| Bulky disease | ||||
| Yes | 27 (31) | 18 (29) | 9 (36) | .609 |
| No | 61 (69) | 45 (71) | 16 (64) | |
| Bone marrow involvement | ||||
| Yes | 20 (23) | 15 (24) | 5 (20) | .785 |
| No | 68 (77) | 48 (76) | 20 (80) | |
| Spleen involvement | ||||
| Yes | 14 (16) | 11 (17) | 3 (12) | .749 |
| No | 74 (84) | 52 (83) | 22 (88) |
| Characteristic . | All patients (n = 88), no. (%) of patients . | I-PET negative (n = 63), no. (%) of patients . | I-PET positive (n = 25), no. (%) of patients . | P . |
|---|---|---|---|---|
| Age | ||||
| > 60 y | 35 (40) | 28 (44) | 7 (28) | .227 |
| < 60 y | 53 (60) | 35 (56) | 18 (72) | |
| Sex | ||||
| Female | 47 (53) | 29 (46) | 18 (72) | .034 |
| Male | 41 (47) | 34 (54) | 7 (28) | |
| Ann Arbor stage | ||||
| III or IV | 59 (67) | 37 (59) | 22 (88) | .011 |
| I or II | 29 (33) | 26 (41) | 3 (12) | |
| ECOG Performance Status | ||||
| > 2 | 25 (28) | 15 (24) | 10 (40) | .189 |
| 0 to 1 | 63 (72) | 48 (76) | 15 (60) | |
| LDH | ||||
| > 450 U/L | 42 (48) | 28 (44) | 14 (56) | .354 |
| < 450 U/L | 46 (52) | 35 (56) | 11 (44) | |
| Extranodal involvement | ||||
| > 2 | 23 (26) | 13 (21) | 10 (40) | .104 |
| 0 or 1 | 65 (74) | 50 (79) | 15 (60) | |
| Age-adjusted IPI risk | ||||
| > 3 | 35 (40) | 23 (37) | 12 (48) | .344 |
| 0-2 | 53 (60) | 40 (63) | 13 (52) | |
| Bulky disease | ||||
| Yes | 27 (31) | 18 (29) | 9 (36) | .609 |
| No | 61 (69) | 45 (71) | 16 (64) | |
| Bone marrow involvement | ||||
| Yes | 20 (23) | 15 (24) | 5 (20) | .785 |
| No | 68 (77) | 48 (76) | 20 (80) | |
| Spleen involvement | ||||
| Yes | 14 (16) | 11 (17) | 3 (12) | .749 |
| No | 74 (84) | 52 (83) | 22 (88) |
A total of 264 PET scans was performed and centrally reviewed, and concordance in the reading among centers was 96%. Sixty-three patients (72%) were negative and 25 (28%) positive at I-PET; 77 patients (88%) were negative and 11 (12%) positive at F-PET. Fifteen of 25 (60%) I-PET positive patients converted to negative at F-PET, whereas only 1 of 63 (2%) I-PET negative cases had a positive F-PET (Table 2). At the end of therapy, 79 patients (90%) achieved a CR and 9 (10%) were nonresponders. The concordance between clinical CR and F-PET negativity was 97%: 2 patients, while in CR, had false positive final scans because of parotid and colorectal cancer (histologically confirmed). At the time of this analysis, 11 patients relapsed or progressed despite I-PET negativity and 9 patients with I-PET positive failed; positive predictive value (PPV) and negative predictive value (NPV) of the I-PET by progression were 36.0% and 82.5%, respectively, with a sensitivity of 45% and a specificity of 76.5%.
Correlation between I-PET and F-PET results
| . | 88 patients . | F-PET negative, no. (%) . | F-PET positive, no. (%) . |
|---|---|---|---|
| I-PET overall | |||
| I-PET negative | 63 | 62 (98.4) | 1 (1.6) |
| I-PET positive | 25 | 15 (60.0) | 10 (40.0) |
| I-PET after 2 courses | 58 | ||
| I-PET negative | 39 | 39 (100) | 0 (0) |
| I-PET positive | 19 | 12 (63.2) | 7 (36.8) |
| I-PET after 3 or 4 courses | 30 | ||
| I-PET negative | 24 | 23 (95.8) | 1 (4.2) |
| . | 88 patients . | F-PET negative, no. (%) . | F-PET positive, no. (%) . |
|---|---|---|---|
| I-PET overall | |||
| I-PET negative | 63 | 62 (98.4) | 1 (1.6) |
| I-PET positive | 25 | 15 (60.0) | 10 (40.0) |
| I-PET after 2 courses | 58 | ||
| I-PET negative | 39 | 39 (100) | 0 (0) |
| I-PET positive | 19 | 12 (63.2) | 7 (36.8) |
| I-PET after 3 or 4 courses | 30 | ||
| I-PET negative | 24 | 23 (95.8) | 1 (4.2) |
Predictive values: NPV, 82.5%; PPV, 36%; specificity, 76.5%; and sensitivity, 45%.
NPV indicates negative predictive value; and PPV, positive predictive value.
We also analyzed the possible correlation between I-PET performed after 2 versus 3 or 4 courses of chemotherapy and F-PET. Fifty-eight patients had I-PET performed after 2 courses: all 39 patients negative at the I-PET remained negative at the F-PET, and 12 of 19 patients positive at the I-PET converted to negative at F-PET. Thirty patients had I-PET performed after 3 (9 patients) or 4 (21 patients) courses of chemotherapy: 23 of 24 patients I-PET negative remained negative at the F-PET, whereas only one patient had a positive F-PET; conversely, 3 of 6 patients I-PET positive converted to negative at F-PET (Table 2).
A δSUV analysis between baseline and I-PET or between baseline and F-PET was also performed during the central review process on 46 patients. At baseline, the median SUVmax was 16.935 (range, 5.990-33.71). At the time of I-PET evaluation, the median SUVmax was 2.405 (range, 1.220-20.000) corresponding to a median basal-interim δSUVmax of 83.1% (range, 18.2%-94.5%). At the time of F-PET evaluation, the median SUVmax was 2.075 (range, 0.960-19.140) corresponding to a median basal-final δSUVmax of 86.9% (range, 22.1%-95%).
A median basal-interim δSUVmax was 80.3% (range, 19%-91.4%) in progressed patients and 84.1% (range, 18.2%-94.5%) in patients without progression, respectively (Mann-Whitney, P = .113). The more than 66% basal-interim δSUVmax was 84.1% in patients without progression and 80.3% in failure patients (P = .113). The 2-year PFS was 68% in less than 66% BI-δ SUVmax and 87% in more than 66% BI-δ SUVmax subgroups, respectively (P = .144).
Treatment outcome
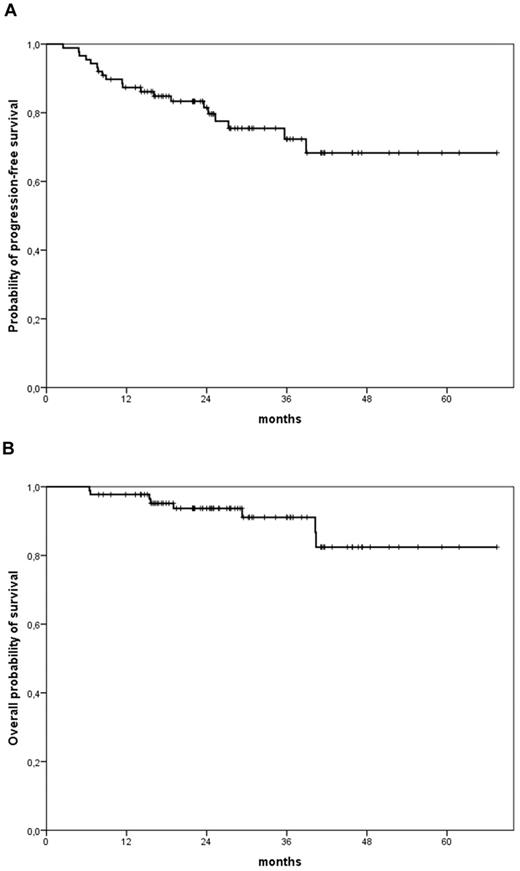
With a median follow-up of 26.2 months, 2-year OS and 2-year PFS were 91% and 77%, respectively, for the whole series of patients (Figure 1). There was a weak correlation between PFS and I-PET results, with a minor difference in 2-year PFS rates between I- PET negative and positive patients: 85% for negative and 72% for positive patients (P = .047; Figure 2A). Conversely, F-PET strongly predicted 2-year PFS (P < .001): 83% for negative and 64% for positive patients, respectively (Figure 2B). In univariate analyses with PFS as outcome measure, elevated LDH value, more than or equal to 2 extranodal sites, bone marrow involvement, intermediate-high/high IPI score, and I-PET and F-PET positivity were predictors of lower PFS rates. The use of G-CSF or number of R-CHOP courses before I-PET did not influence PFS rates (Tables 3 and 4). Two independent bivariate analyses by Cox models were performed to properly evaluate the prognostic role of I-PET and F-PET results for PFS. In model 1, only F-PET retained its prognostic value compared with I-PET with an adjusted hazard ratio (HR) of 5.03 (95% CI, 1.37-18.43, P = .015) versus 1.27 (95% CI, 0.40-4.03, P = .691). In model 2, both F-PET (HR = 4.54, 95% CI, 1.68-12.31) and IPI score (HR = 5.36, 95% CI, 1.91-15.05, P = .001) remained independent prognostic factors for PFS (Table 4).
PFS and OS for entire patient population. (A) Two-year PFS, 77%. (B) Two-year OS, 91%.
PFS and OS for entire patient population. (A) Two-year PFS, 77%. (B) Two-year OS, 91%.
PFS according to response at I-PET and F-PET. (A) PFS by I-PET, 85% negative patients (solid line) and 72% positive patients (dashed line), respectively (P = .047). (B) PFS by F-PET, 83% negative patients (solid line) and 64% positive patients (dashed line), respectively (P < .001).
PFS according to response at I-PET and F-PET. (A) PFS by I-PET, 85% negative patients (solid line) and 72% positive patients (dashed line), respectively (P = .047). (B) PFS by F-PET, 83% negative patients (solid line) and 64% positive patients (dashed line), respectively (P < .001).
Univariate analyses (Cox model) of progression
| . | Hazard ratio . | 95% CI . | P . |
|---|---|---|---|
| Sex (female vs male) | 1.06 | 0.44-2.56 | .895 |
| Age (> 60 y vs ≤ 60 y) | 2.04 | 0.84-4.92 | .114 |
| Stage (III or IV vs I or II) | 2.51 | 0.84-7.52 | .101 |
| ECOG PS (≥ 2 vs 0 or 1) | 2.15 | 0.89-5.21 | .089 |
| LDH (> 450 U/L vs ≤ 450 U/L) | 6.16 | 2.04-18.51 | .001 |
| Extranodal involvement (≥ 2 vs 0 or 1) | 2.70 | 1.11-6.53 | .028 |
| aaIPI (≥ 3 vs 0-2) | 6.05 | 2.18-16.82 | .001 |
| Bulky disease (yes vs no) | 1.63 | 0.67-3.99 | .286 |
| Bone marrow involvement (yes vs no) | 2.41 | 0.98-5.89 | .055 |
| Spleen involvement (yes vs no) | 1.97 | 0.71-5.42 | .191 |
| Radiotherapy (yes vs no) | 0.57 | 0.13-2.45 | .448 |
| G-CSF administration (yes vs no) | 2.39 | 0.32-18.14 | .398 |
| No. of R-CHOP cycles at I-PET (3 or 4 vs 2) | 1.06 | 0.42-2.67 | .901 |
| R-CHOP cycle interval (21 vs 14) | 1.07 | 0.44-2.63 | .878 |
| I-PET result (positive vs negative) | 2.45 | 1.01-5.93 | .047 |
| F-PET result (positive vs negative) | 5.97 | 2.19-16.28 | < .001 |
| Clinical response (no CR vs CR) | 14.28 | 5.26-49.88 | < .001 |
| . | Hazard ratio . | 95% CI . | P . |
|---|---|---|---|
| Sex (female vs male) | 1.06 | 0.44-2.56 | .895 |
| Age (> 60 y vs ≤ 60 y) | 2.04 | 0.84-4.92 | .114 |
| Stage (III or IV vs I or II) | 2.51 | 0.84-7.52 | .101 |
| ECOG PS (≥ 2 vs 0 or 1) | 2.15 | 0.89-5.21 | .089 |
| LDH (> 450 U/L vs ≤ 450 U/L) | 6.16 | 2.04-18.51 | .001 |
| Extranodal involvement (≥ 2 vs 0 or 1) | 2.70 | 1.11-6.53 | .028 |
| aaIPI (≥ 3 vs 0-2) | 6.05 | 2.18-16.82 | .001 |
| Bulky disease (yes vs no) | 1.63 | 0.67-3.99 | .286 |
| Bone marrow involvement (yes vs no) | 2.41 | 0.98-5.89 | .055 |
| Spleen involvement (yes vs no) | 1.97 | 0.71-5.42 | .191 |
| Radiotherapy (yes vs no) | 0.57 | 0.13-2.45 | .448 |
| G-CSF administration (yes vs no) | 2.39 | 0.32-18.14 | .398 |
| No. of R-CHOP cycles at I-PET (3 or 4 vs 2) | 1.06 | 0.42-2.67 | .901 |
| R-CHOP cycle interval (21 vs 14) | 1.07 | 0.44-2.63 | .878 |
| I-PET result (positive vs negative) | 2.45 | 1.01-5.93 | .047 |
| F-PET result (positive vs negative) | 5.97 | 2.19-16.28 | < .001 |
| Clinical response (no CR vs CR) | 14.28 | 5.26-49.88 | < .001 |
Bivariate analyses (Cox model) of progression
| . | Hazard ratio . | 95% CI . | P . |
|---|---|---|---|
| Model 1 | |||
| I-PET result (positive vs negative) | 1.27 | 0.40-4.03 | .691 |
| F-PET result (positive vs negative) | 5.03 | 1.37-18.43 | .015 |
| Model 2 | |||
| aaIPI (≥ 3 vs 0-2) | 5.36 | 1.91-15.05 | .001 |
| F-PET result (positive vs negative) | 4.54 | 1.68-12.31 | .003 |
| . | Hazard ratio . | 95% CI . | P . |
|---|---|---|---|
| Model 1 | |||
| I-PET result (positive vs negative) | 1.27 | 0.40-4.03 | .691 |
| F-PET result (positive vs negative) | 5.03 | 1.37-18.43 | .015 |
| Model 2 | |||
| aaIPI (≥ 3 vs 0-2) | 5.36 | 1.91-15.05 | .001 |
| F-PET result (positive vs negative) | 4.54 | 1.68-12.31 | .003 |
Discussion
The aim of our study was to determine the predictive value of I-PET and F-PET on the outcome in a series of DLBCL patients homogeneously treated with R-CHOP chemotherapy. In our population, 40% of patients had a poor-risk IPI score, according to different reported DLBCL prognostic subgroup rates.2 Our results confirmed, as in other series, the strong predictive value of the F-PET on PFS. On the contrary, I-PET failed to clearly identify patients with different PFS rates. Although in our series of patients a negative interim 18F-FDG-PET/CT scan predicted a good outcome with a 2-year PFS of 85%, a positive I-PET scan was not able to identify patients with a worse prognosis: notably, in our study, positive I-PET patients had a only slightly inferior 2-year PFS (72%). Indeed, the negative predictive value for I-PET was 82.5%, but positive predictive value of I-PET was only 36.0%. To better analyze the predictive value of I-PET and F-PET, we performed 2 independent bivariate analyses by Cox models. The results of both models underlined that only F-PET and IPI score remained independent prognostic factors for PFS (Tables 3 and 4), whereas I-PET had a very weak correlation with the outcome.
Only a portion of patients with DLBCL are cured by standard chemotherapy with rituximab.13,14 Salvage high-dose chemotherapy followed by autologous stem cell transplantation may rescue some patients with refractory or relapsed disease. However, the chance to salvage refractory/relapsed patients previously treated with R-CHOP is nowadays largely unsatisfactory with less than 30% to 35% of these patients being cured even after high-dose chemotherapy followed by autologous stem cell transplantation.1,17 The upfront use of this intensive approach in poor prognosis patients is controversial with different results among published reports,18–20 and it is not recommended outside of clinical trials because a proportion of patients may experience a long PFS with R-CHOP.
Therefore, the possibility to find a tool that is able to predict an unfavorable outcome early during the treatment of these patients is an attractive option and may have important clinical implications. 18F-FDG-PET/CT plays a defined role in staging and restaging of DLBCL and Hodgkin lymphoma (HD) and has been incorporated into the International Workshop Criteria for determining final response.5,8,21 A negative interim restaging PET scan was also reported to be associated with a good outcome, but there is no consensus about the results regarding positive I-PET scan during therapy.
Former preliminary studies showed that metabolic changes after one to 3 courses of chemotherapy may be highly predictive of final response and PFS.7,16,22,23 However, Terasawa et al published a meta-analysis of 13 different studies, including 311 DLBCL and 360 HD patients.22 The DLBCL population was heterogeneous regarding prognosis and therapies; often, studies had a small number of patients included, and there was a wide variability in the number of cycles before PET scan and in the time after the cycle chosen for the I-PET performing. A potential limit of our study may be a not so large sample size or I-PET performed at different time points. However, our population included a clinically homogeneous cohort of 88 DLBCL patients, consecutively observed, and all treated with standard R-CHOP. In our series, I-PET was usually performed after 2 courses of chemotherapy and only in one-third of patients after 3 or 4 courses. Regardless of the different time points (after 2 vs 3 or 4 courses of chemotherapy), I-PET was not able to identify subgroups of patients with different outcomes. Recently, in a study published by Zinzani et al on aggressive non-Hodgkin lymphoma patients treated with immunochemotherapy, the positive midtreatment PET appeared to identify a group of patients with a worse outcome in terms of PFS and OS.24 Conversely, Moskowitz et al showed that, in case of I-PET positivity in DLBCL patients treated with a dose-dense (sequential) immunochemotherapy, only a confirmatory biopsy can allow a change of treatment because most patients with positive I-PET scans had negative histologic specimens.25 Finally, there was a large variation in I-PET results, and there were not consistent and standardized criteria of the PET reading. Recently, Horning et al reported only a moderate reproducibility (68%) among the nuclear medicine experts in I-PET interpretation.26 To avoid this bias in our study, all PET scans were centrally reviewed by 2 different nuclear medicine experts according to the First Consensus Conference.15 These resulting Juweid criteria4 are the established method for end-of therapy evaluation in lymphoma and are designed to achieve the best possible safety in defining a patient as negative at the end of therapy; on the other hand, the Deauville system for interim evaluation is flexible and gives the possibility to change the cut-off value depending on different factors (histology, number of chemotherapy courses, endpoint of different protocols).
As DLBCL patients respond to therapy in a continuous modality, with a relatively higher rate of minimal residual uptake compared with HD patients at interim evaluation, we chose to set the cut-off value at grade 4 of the 5-point score; indeed, by considering the liver as reference background, the system tends to be more “forgiving” (ie, have less false positives). In our study, we willingly chose to use 2 different methods of evaluation, considering that the cellular kinetics and the inflammatory component weigh differently at the 2 different time points.
Meignan et al suggested that a quantitative approach based on SUVmax reduction between baseline PET and I-PET may have a higher predictive value than visual analysis when PET was performed at 2 cycles but was equivalent to visual analysis at 4 cycles.27 Therefore, it appears that interpretation criteria of I-PET are far from being properly defined for the evaluation of tumor response and chemosensitivity. Moreover, in a more recent study published by Casasnovas et al, the visual analysis of I-PET was compared with δSUV analysis: the first one was not able to predict a different outcome in positive or negative population, but a δSUV evaluation with a cut-off of 66% SUVmax reduction identified patients with different PFS and OS, mainly if performed after 4 courses of chemotherapy.28 This conclusion might mean that, in DLBCL patients, the response to chemotherapy requires time to be revealed. In our study, the δSUV analysis between baseline and I-PET or between baseline and F-PET performed on a subgroup of 46 patients did not evidence a contribution of this factor on predicting different PFS. It would appear that patients with a higher δ SUVmax follow the trend of a good outcome, but our data cannot define the contrary in those with a less marked δSUVmax at interim evaluation. Quantitative analysis or response in DLBCL may be a promising tool in the near future, but to date it needs further validation in clinical prospective studies conducted in wide homogeneous populations and with internationally validated reporting criteria.
There are some potential explanations for false-positive scans. FDG as a marker does not have such a high specificity because it is also taken up in infections and inflammatory processes.29–31 Moreover, it is also possible that the use of immunotherapy may increase lesion inflammation; in addition, antibody-mediated cellular cytotoxicity and complement activation are important mechanisms in rituximab's activity. Both processes are able to attract mediators of inflammation to tumor site. The variable use of rituximab in previous studies between none7,10 and in a minority of patients (41%)32 compared with the use in all patients may explain a higher rate of I-PET positivity unrelated to tumor activity in our study.33 Another important feature to consider is whether the timing of PET scan during treatment affects the results. According to the recommendations by the European Organization for Research and Treatment of Cancer, a time interval of one to 2 weeks between a completion of chemotherapy cycle and FDG-PET scans is considered optimal to avoid transient flare at the disease sites.34 In our study, we performed the I-PET scans at a median time of 13 days after the previous course of chemotherapy and the F-PET scans more than 21 days (median time, 36 days) from the previous R-CHOP course in all patients to avoid this interference pattern.
So, at this time, there is no clear evidence for supporting I-PET outside of clinical trials because a positive scan during treatment does not mean failure in a single patient as reported by Cheson et al, and physicians should not change therapy for lymphoma based on an I-PET in practice.12
Our study, with the limits of a not so large sample size and a different time point analysis, was addressed to analyze the prognostic value of I-PET on the outcome in a completely homogeneous cohort of DLBCL patients in terms of clinical characteristics and therapy used.
In conclusion, our data would indicate that, in DLBCL patients undergoing R-CHOP as first-line treatment, positive I-PET scans did not identify high-risk patients with a worse outcome, whereas only negative I-PET results seem to predict a favorable outcome. Moreover, the F-PET scans confirmed its high predictive value for PFS. Larger prospective trials and optimization and standardization of criteria for I-PET evaluation for DLBCL patients are needed to assess the real prognostic value of I-PET results.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported in part by Ministero della Salute, Dipartimento dell'Innovazione-Direzione Generale Ricerca Scientifica e Tecnologica (unrestricted grant, Progetto di Ricerca Finalizzata 2008, Istituto di Ricovero e Cura a Carattere Scientifico Centro di Rifermento Oncologico della Basilicata Rionero CUP J65J08000090000).
Authorship
Contribution: P.P., A.C., M.L., and U.V. conceived and designed the study; P.P., A.C., M.B., B.B., S. Ferrero, S. Franceschetti, F.G., G.L., M.M., M.N., B.P., L.R., F.S., and L.V. provided study materials or patients; P.P., A.C., G.P., and R.P. collected and assembled data; P.P., A.C., M.B., M.L., R.P., G.B., and U.V. analyzed and interpreted data; and P.P., A.C., and U.V. wrote the manuscript.
Conflict-of-interest disclosure: U.V. was on the advisory committee of Roche Italy and received a lecture fee from Roche. The remaining authors declare no competing financial interests.
Correspondence: Patrizia Pregno, Hematology 2, San Giovanni Battista Hospital, Corso Bramante 88, 10126 Turin, Italy; e-mail: ppregno@molinette.piemonte.it.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal