Abstract
The C-type lectin receptor CLEC-2 signals through a pathway that is critically dependent on the tyrosine kinase Syk. We show that homozygous loss of either protein results in defects in brain vascular and lymphatic development, lung inflation, and perinatal lethality. Furthermore, we find that conditional deletion of Syk in the hematopoietic lineage, or conditional deletion of CLEC-2 or Syk in the megakaryocyte/platelet lineage, also causes defects in brain vascular and lymphatic development, although the mice are viable. In contrast, conditional deletion of Syk in other hematopoietic lineages had no effect on viability or brain vasculature and lymphatic development. We show that platelets, but not platelet releasate, modulate the migration and intercellular adhesion of lymphatic endothelial cells through a pathway that depends on CLEC-2 and Syk. These studies found that megakaryocyte/platelet expression of CLEC-2 and Syk is required for normal brain vasculature and lymphatic development and that platelet CLEC-2 and Syk directly modulate lymphatic endothelial cell behavior in vitro.
Introduction
Recently, several mutant mouse models have shown a defect in the separation of the lymphatic vasculature from the blood vasculature typically resulting in the appearance of blood-filled lymphatic vessels in the skin at embryonic day (E) 14.5 (review in Tammela and Alitalo1 ). Mice deficient in the tyrosine kinase Syk show this phenotype during gestation and die around the time of birth.2-4 A similar defect is found in mice deficient in the adapter protein SLP76 (Lcp2)4 or in PLCγ2,5 which play vital roles downstream of Syk in immunoreceptor tyrosine-based activation motif (ITAM) and integrin signaling cascades, providing circumstantial evidence that the Syk-SLP76-PLCγ2 pathway is required for normal lymphatic development.
The C-type lectin-like protein type 2 (CLEC-2, encoded by the Clec1b gene) is highly expressed on platelets and at lower levels on other hematopoietic cells6-9 and signals through a cytosolic YxxL sequence known as a hemITAM.10,11 These receptors signal through a similar pathway used by ITAM receptors which have a dual YxxL/I sequence. HemITAM receptors activate Syk, initiating a signaling cascade partially dependent on SLP76 that leads to activation of PLCγ2.6,12,13 The role of CLEC-2 in hemostasis and thrombosis is debatable because some lines of evidence suggest that it is required14,15 and others show that it has no significant involvement in these processes.16
CLEC-2 has been recognized as a receptor for the transmembrane protein podoplanin.17,18 Podoplanin is expressed on lymphatic endothelial cells (LECs), lung type-1 alveolar cells, and kidney podocytes but not in blood endothelial cells (BECs). Podoplanin-deficient mice die shortly after birth because of an inability to inflate their lungs and, like Syk-deficient mice, show dilated, tortuous blood-filled lymphatics in mid-gestation.19,20 A similar phenotype is seen in mice lacking megakaryocytes/platelets.21 A series of recent studies has shown that deletion of CLEC-2 resulted in blood-filled lymphatics and vascular defects in mid-gestation and in perinatal lethality in most offspring.15,16,22,23 Furthermore, the same phenotype was observed after the targeted disruption of SLP76 in the megakaryocyte/platelet lineage by crossing SLP76fl/fl mice to PF4-Cre transgenic mice,22 although this strategy also induces a limited excision in a subpopulation of other hematopoietic cells.24 Furthermore, it is notable that CLEC-2 signaling partially depends on this adaptor protein, and constitutive SLP76-deficient mice are viable in contrast to CLEC-2–deficient mice.4,6 Together, these studies indicated that separation of the lymphatics from blood vessels requires podoplanin and CLEC-2 signaling.
These data support a model in which Syk and SLP76-dependent platelet activation through engagement of CLEC-2 by podoplanin (presumably on LECs) is essential for separation of LECs from the cardinal vein in mid-gestation. However, these studies do not address whether other cells contribute to this phenotype, because both CLEC-2 and Syk are expressed elsewhere in the hematopoietic system.9,25 Moreover, it has been suggested that defective lymphatics in Syk- and SLP76-deficient mice may be caused by loss of key functions for both proteins in endothelial precursors.26 Another proposal is that the lymphatic defect in Syk-deficient mice may be because of loss of the kinase in macrophages.27 None of these studies, however, provide an insight into the underlying mechanism by which platelets contribute to the normal development of lymphatics and do not explain the high level of perinatal morbidity of CLEC-2– and Syk-deficient mice.
To investigate the role of CLEC-2 and Syk in the development of the embryo, we analyzed mice constitutively deficient in CLEC-2 and Syk throughout gestation and compared these with mice with a selective deletion of CLEC-2 and Syk in several lineages. We have also investigated the effects of platelets on LEC behavior in vitro.
Methods
Mouse strains
All animal experimentation was performed under an approved license from the UK Home Office. CLEC-2–deficient mice (Clec1b−/−) and radiation chimeras reconstituted with Clec1b−/− fetal liver cells have been previously described.16 Conditional deletion of CLEC-2 was achieved by insertion of loxP sites flanking exons 3 and 4 of the Clec1b gene (Clec1bfl/fl), using standard methods. Cre-mediated recombination of the Clec1bfl allele results in deletion of exons 3 and 4, and a frameshift in exons 5 and 6. Syk-deficient mice (Syktm1Tyb/tm1Tyb, Syk−/−) on a C57BL/6J background were described earlier.2 Mice carrying conditional alleles of Syk (Sykfl/fl) had loxP sites introduced flanking exon 11 (N.S. and S.S., manuscript in preparation) of the Syk gene. PF4Cre, Vav-iCre, hCD2-iCre, LysMCre, and Tie1Cre transgenic mice have been described previously.28-32 Sykfl/fl-CD11c mice were generated by breeding C57BL/6-Sykfl/fl33 (gift from Alexander Tarakhovsky, Rockefeller University) and CD11c-Cre (B6.Cg-Tg [Itgax-cre] 1-1Reiz) mice.32 Genotyping was performed by PCR with the use of genomic DNA isolated from tail/ear tissue with primers listed in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Histologic analyses
Time-mated females (day of plug = E0.5), and embryos were culled by Schedule 1 procedures. P0 lungs were inflated with 50μL of PBS and fixed in 10% neutral buffered formalin (NBF) overnight, as were E12.5 heads. All tissues were processed to 5-μm paraffin sections stained with H&E. P0 lung sections were stained for podoplanin (Angiobio) localization with visualization with the use of the Vectastain ABC kit (Vector Laboratories) and counterstained with Harris Hematoxylin (Sigma-Aldrich), dehydrated, and mounted with Vectamount (Vector Laboratories). Sections were photographed by Axiocam (Zeiss) in brightfield illumination at 10× and 63× on an Axiovert 200M (Zeiss).
Isolation of cells
Mesenteric vessels and intestine were disaggregated by incubation in 2.5 mg/mL collagenase/dispase (Roche), 100 μg/mL DNase I (Sigma-Aldrich) in RF10 media at 37°C for 30 minutes. Lungs were disaggregated mechanically and digested in sequential incubations (45 minutes and 20 minutes) with 2.5 mg/mL collagenase D, 0.2 mg/mL DNase I and 2.5 mg/mL collagenase dispase, 100 μg/mL DNase I in 2% FCS RPMI media at 37°C. Single-cell suspensions were obtained by pipetting. Final incubation was in 5mM EDTA for 5 minutes at 37°C before the suspension was filtered through 40-μm cell strainers. Cells were then washed and resuspended in MACS buffer for staining.
Flow cytometry
The following Abs were used for flow cytometry: CD31-FITC clone 390, gp38/podoplanin-PE clone eBio8.1.1, Ter-119–peridinin chlorophyll protein complex–cyanine 5.5 clone Ter-119, and CD45 APC clone 30-F11 (eBioscience). Four-color flow cytometric analysis was performed with the use of FACSCalibur (BD Biosciences). Data were analyzed with FlowJo Version 8.8.6 software (TreeStar) and presented as the ratio of the percentage of LECs (podoplanin+, CD31+) counted per the percentage of BECs (podoplanin−, CD31+) counted in the stromal fraction (CD45−, Ter119−) of each cell preparation.
FITC-dextran injection
For all studies FITC-dextran (150 kDa; Sigma-Aldrich) was used at a concentration of 25 mg/mL in PBS. For kinetic studies on Clec1bfl/fl animals, mice were anesthetized, and mesenteric circulation was visualized with bright field microscopy (Olympus BX-061WI). Recording at 50-millisecond intervals with the use of Slidebook 5 (Intelligent Imaging Innovations) was started ∼ 20-30 seconds before FITC-dextran infusion through a carotid cannula. Flow through the mesenteric vessels was recorded for ≥ 3 minutes. Studies on Sykfl/fl animals were performed by injecting FITC-dextran (150 μL) into the left ventricle of the heart immediately after cervical dislocation. After 60 seconds the mesentery was visualized with Zeiss Stemi SV11 microscope equipped with a Hamamatsu C4742 camera on Openlab Version 4 software (Perkin-Elmer).
Lymphatic endothelial cell transmigration and network formation assays
The effect of platelet-expressed CLEC-2 on LEC migration was assessed with the transfilter assay. Cell culture polyethylene terephthalate inserts (BD Biosciences) with 8-μm pores were placed in 24-well plates and complete growth medium MV2 (Promocell) containing 350 ng/mL VEGF-C (R&D Systems) in the lower wells. Human LECs (HLECs; Promocell) were nonenzymatically detached, resuspended in MV2, and plated on top at 3 × 104 cells/insert.
Whole mouse blood was drawn into acid citrate dextrose (1/9 vol) from CO2-asphyxiated mice after isofluorane anesthesia. Washed platelet suspensions were obtained by centrifugation and resuspended as previously described.13,16 Platelets (108), Tyrode buffer, or platelet releasate from rhodocytin-stimulated (300nM) 108 platelets was added 1 hour after seeding and incubated for 18 hours at 37°C, 5% CO2. Podoplanin cross-linking was achieved by treating LECs with 2 μg/mL of the rat-anti–human podoplanin Ab NZ-1.3 (eBioscience), plus a cross-linking anti–rat IgG2a Ab (Biolegend) at a 1:15 ratio. Negative controls contained 2 μg/mL rat IgG with and without anti–rat IgG2a. Cells attached to the insert membrane were washed with PBS, fixed with 2% formaldehyde, and stained with 2 μg/mL bisbenzimide (Sigma-Aldrich). The stained nuclei were visualized with an AxioVert 200M inverted fluorescent microscope (Zeiss), and the numbers of cells above and below the filter were counted in 20 fields/insert. Percentage of transmigration was calculated as the number of migrated cells/total number of cells.
The network formation assay was performed on 12-well plates coated with 100 μL Matrigel (BD Biosciences) diluted at 6 mg/mL in culture medium. After polymerization, HLECs (2 × 105) resuspended in 2 mL of MV2 were added to each well and incubated at 37°C, 5% CO2 for 2 hours. The medium was changed, and 200 μL of Tyrode buffer, washed platelet suspension (2.5 × 108 platelets/mL), or platelet releasate from rhodocytin-stimulated (300nM) 108 platelets was added to the wells.
The effect of platelets on network formation was evaluated 3 hours after application of platelets (5 hours after endothelial cell seeding) with the use of an inverted microscope (Olympus) at 4× magnification. Total tube length in the resulting images (5 fields/well) was blindly quantified with ImageJ Version 1.42q software (National Institutes of Health).34
Statistical analyses
Numbers of mice obtained from transgenic lines were subjected to chi-square test. Ratio of LECs to BECs is presented as mean ± SEM. Transmigration and tube-forming assay numbers are presented as mean ± SD. All data were tested with 2-tailed unpaired t tests in GraphPad Prism Version 4 software, and differences were considered significant when P ≤ .05.
Results
CLEC-2–deficient mice show hallmarks of defective lymphatic development and function
Up to P0, the number of Clec1b−/− offspring from Clec1b+/− timed matings was at Mendelian frequency (Table 1). In contrast, only 3 Clec1b−/− offspring survived beyond P10 from a total of 205 offspring. These 3 survived < 30 days postpartum and had to be humanely killed because of their deteriorating condition. These results are similar to those described for other constitutive Clec1b−/− models.15,22,23
Offspring resulting from Clec1b+/+ matings
| Stage . | Total number of mice . | Number of Clec1b−/− mice . | |
|---|---|---|---|
| Expected . | Found . | ||
| E10.5 | 70 | 17.5 | 14 |
| E12.5 | 79 | 19.75 | 19 |
| E14.5 | 45 | 11.25 | 11 |
| E16.5 | 44 | 11 | 7 |
| E18.5 | 16 | 4 | 5 |
| P0 | 40 | 10 | 7 |
| Up to P30 | 205 | 38.75 | 3* |
| Stage . | Total number of mice . | Number of Clec1b−/− mice . | |
|---|---|---|---|
| Expected . | Found . | ||
| E10.5 | 70 | 17.5 | 14 |
| E12.5 | 79 | 19.75 | 19 |
| E14.5 | 45 | 11.25 | 11 |
| E16.5 | 44 | 11 | 7 |
| E18.5 | 16 | 4 | 5 |
| P0 | 40 | 10 | 7 |
| Up to P30 | 205 | 38.75 | 3* |
Time-mated Clec1b+/− females were killed, and the resultant offspring were genotyped. Shown are the expected and actual numbers of Clec1b−/− offspring.
Significant reduction of mice found by χ2 test (P < .005).
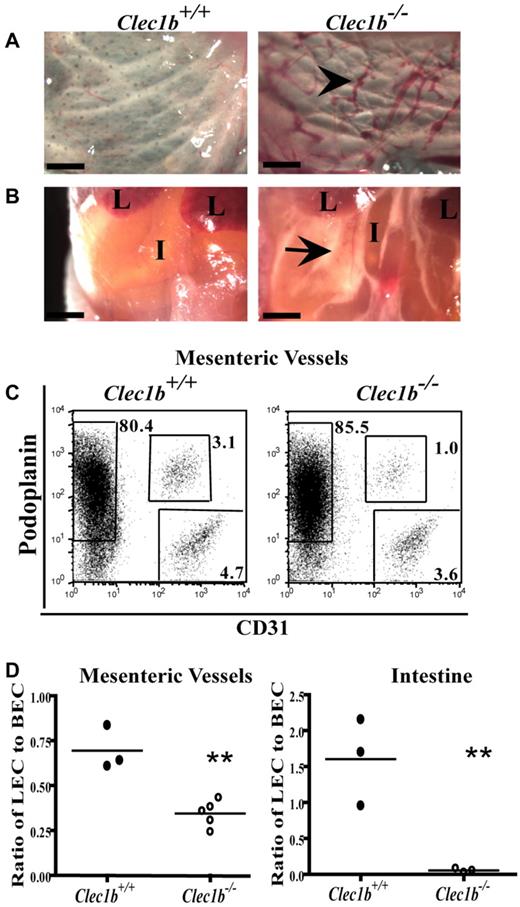
Clec1b−/− embryos showed numerous, blood-filled vessels in the skin, confirmed to be lymphatic in origin by expression of LYVE-1, from E14.5 to E18.5 with no evidence of this before E14.5. Clec1b−/− embryos presented with edematous swelling throughout their back and limbs from E14.5 onward. At birth, Clec1b−/− offspring are readily distinguishable from their wild-type littermates because of their edematous swelling and signs of severe respiratory distress with > 90% of the animals surviving < 24 hours. In addition, occasional discreet hemorrhages were seen in the skin (supplemental Figure 1). All Clec1b−/− offspring had blood-filled vessels in their skin (Figure 1A). Most Clec1b−/− offspring did not survive long enough to feed, but those that did developed chylous ascites in the abdomen (Figure 1B), a further indication of defective lymphatic function.
Clec1b−/− newborn pups are nonviable and visually distinct from Clec1b+/+ littermates at birth. (A) Blood-filled lymphatic vessels (black arrowhead) in the subcutaneous region of the skin persist at P0 (scale bar = 500 μm). (B) Clec1b−/− newborn pups that feed develop chylous ascites (black arrow) in the abdominal cavity (L indicates lobes of the liver; I, intestine; scale bar = 1 mm). (C) Example of flow cytometric analysis identifying LECs (podoplanin+CD31+) and BECs (podoplanin−CD31+) in the stromal fraction (CD45−Ter119−) of mesenteric vessel preparations from Clec1b+/+ (left) and Clec1b−/− (right) offspring. (D) Ratio of LECs to BECs in preparations of isolated mesenteric vessels (left; **P = .002 by unpaired t test; n = 3 Clec1b+/+; n = 5 Clec1b−/−) and intestine (right; **P = .012 by unpaired t test; n = 3 for each genotype) show significant decreases in P0 Clec1b−/− offspring.
Clec1b−/− newborn pups are nonviable and visually distinct from Clec1b+/+ littermates at birth. (A) Blood-filled lymphatic vessels (black arrowhead) in the subcutaneous region of the skin persist at P0 (scale bar = 500 μm). (B) Clec1b−/− newborn pups that feed develop chylous ascites (black arrow) in the abdominal cavity (L indicates lobes of the liver; I, intestine; scale bar = 1 mm). (C) Example of flow cytometric analysis identifying LECs (podoplanin+CD31+) and BECs (podoplanin−CD31+) in the stromal fraction (CD45−Ter119−) of mesenteric vessel preparations from Clec1b+/+ (left) and Clec1b−/− (right) offspring. (D) Ratio of LECs to BECs in preparations of isolated mesenteric vessels (left; **P = .002 by unpaired t test; n = 3 Clec1b+/+; n = 5 Clec1b−/−) and intestine (right; **P = .012 by unpaired t test; n = 3 for each genotype) show significant decreases in P0 Clec1b−/− offspring.
To determine the nature of the lymphatic defect causing the developmental and perinatal phenotype, flow cytometric analysis was performed on isolated mesenteric vessels and intestine of P0 Clec1b−/− mice to identify LECs (CD31+podoplanin+) and BECs (CD31+podoplanin−; Figure 1C). The LEC/BEC ratio was consistently and significantly reduced in the Clec1b−/− offspring (0.35 ± 0.07) in comparison to Clec1b+/+ littermates (0.70 ± 0.07) in the mesenteric vessels surrounding the intestine (Figure 1D). A significant decrease was also seen in the intestine proper of Clec1b−/− mice (0.06 ± 0.02) in comparison to Clec1b+/+ littermates (1.60 ± 0.35). The decreased ratio at P0 may be the result of earlier changes during gestation, because the LEC/BEC ratio is significantly reduced in the mesentery and intestine of Clec1b−/− embryos at both E16.5 and E18.5 (supplemental Figure 2).
Syk plays a critical role in signaling by CLEC-2.6,13 Significantly, many aspects of the Clec1b−/− phenotype, including blood-filled lymphatic vessels in the skin beginning at E14.5, perinatal lethality, and chylous ascites after feeding, have been previously reported for Syk-deficient mice.2-4 Comparison of the blood-filled vessels in Clec1b−/− and Syk−/− embryos showed similar appearance at all times (not shown).
Lung histopathology of P0 Clec1b−/− and Syk−/−
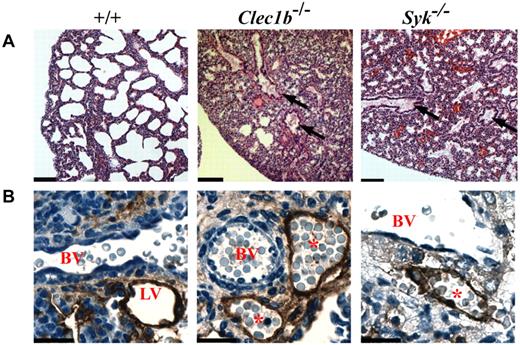
Possibly contributing to the early postnatal lethality of both the Clec1b−/− and Syk−/− pups is the effect of disruption of CLEC-2/Syk signaling on the physiology of the lungs. Clec1b−/− pups found alive after birth always presented with dyspnea. Histologic analysis found a similar morphology in the lungs from Clec1b−/− and Syk−/− mice (Figure 2A). The amount of airspace in both sets of lungs was markedly reduced. Furthermore, in both Clec1b−/− and Syk−/− mice, the terminal air sacs did not appear to be properly inflated, and the larger airways contained fluid droplets. Immunohistochemistry of LECs in sections of P0 lung with the use of an Ab to podoplanin showed that LECs detected in Clec1b−/− and Syk−/− lungs formed a vessel that contained red blood cells (Figure 2B). As with the mesentery and intestine, there is a significant decrease in the ratio of LEC/BEC of > 50% established in Clec1b−/− lungs during development (supplemental Figure 2).
Lungs of P0 Clec1b−/− and Syk−/− mice have a similar pathology. (A) Representative low magnification (10×) photomicrographs of H&E-stained paraffin sections from P0 lung showed detail of the lung's contribution to the lethal phenotype of the Clec1b−/− and Syk−/− mice (n ≥ 3 for each genotype). Lungs from P0 wild-type (+/+) mice showed large open bronchi and expanded terminal air sacs with thin septae between airspaces. In contrast, lungs from Clec1b−/− and Syk−/− mice showed fluid accumulation in the bronchi (black arrows) as well as incompletely expanded terminal airways. Scale bar = 100 μm. (B) Lymphatic vessels (LVs) localized next to blood vessels (BVs) were identified with an Ab to podoplanin (brown staining). These vessels were clear in Clec1b+/+ and Syk+/+ (+/+) mice but contained red blood cells in Clec1b−/− and Syk−/− mice, indicating a connection between the lymphatic and circulatory systems. Scale bar = 20 μm.
Lungs of P0 Clec1b−/− and Syk−/− mice have a similar pathology. (A) Representative low magnification (10×) photomicrographs of H&E-stained paraffin sections from P0 lung showed detail of the lung's contribution to the lethal phenotype of the Clec1b−/− and Syk−/− mice (n ≥ 3 for each genotype). Lungs from P0 wild-type (+/+) mice showed large open bronchi and expanded terminal air sacs with thin septae between airspaces. In contrast, lungs from Clec1b−/− and Syk−/− mice showed fluid accumulation in the bronchi (black arrows) as well as incompletely expanded terminal airways. Scale bar = 100 μm. (B) Lymphatic vessels (LVs) localized next to blood vessels (BVs) were identified with an Ab to podoplanin (brown staining). These vessels were clear in Clec1b+/+ and Syk+/+ (+/+) mice but contained red blood cells in Clec1b−/− and Syk−/− mice, indicating a connection between the lymphatic and circulatory systems. Scale bar = 20 μm.
Loss of CLEC-2 and Syk results in persistent CNS hemorrhage
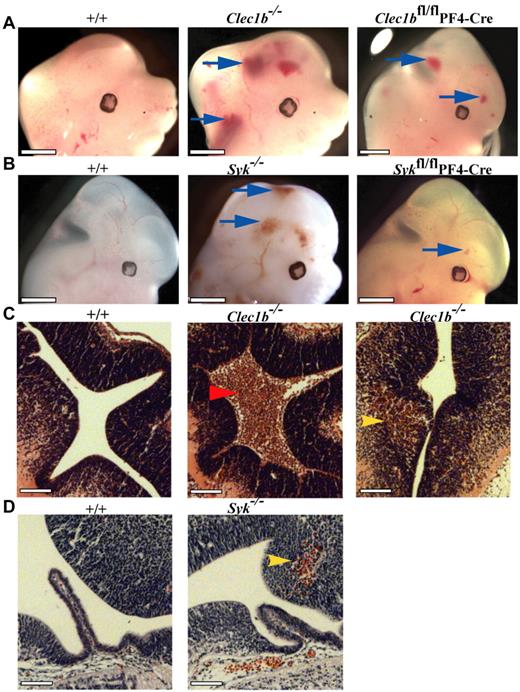
An additional phenotype of hemorrhages within the developing brain and spinal cord at E12.5 was noted in most Clec1b−/− (18 of 19; Figure 3A) and Syk−/− embryos (13 of 14; Figure 3B) persisting through E18.5 (not shown). In Clec1b−/− mice hemorrhages were localized to at least 1 ventricle and the brain parenchyma, whereas in Syk−/− mice they were restricted to the parenchyma (Figure 3C-D). These hemorrhages were never seen in wild-type embryos (n ≥ 15 for each colony; Figure 3A-B). Thus, both CLEC-2 and Syk are required for the integrity of the brain vasculature.
Abnormal hemorrhage within the developing central nervous system of Clec1b−/− and Syk−/− mice from E12.5. (A) Both constitutive (Clec1b−/−; middle; n = 18 of 19) and platelet/megakaryocyte-specific (Clec1bfl/flPF4-Cre; right; n = 3 of 5) ablation of Clec1b results in hemorrhaging within the brain and developing spinal column (blue arrows). (B) Constitutive (Syk−/−; middle; n = 13 of 14) and platelet/megakaryocyte-specific (Sykfl/flPF4-Cre; n = 10 of 13) ablation of Syk presented with a similar hemorrhaging phenotype in similar regions as the Clec1b−/− embryos (blue arrows). None of the littermate wild-type controls (+/+; left; n ≥ 5) showed this phenotype. Scale bars = 1 mm. (C) H&E-stained sections from E12.5 brains show that hemorrhages in Clec1b−/− embryos were localized to at least 1 ventricle (middle; red arrowhead) and within the parenchyma (right; yellow arrowhead). (D) Sections from E12.5 Syk−/− embryos showed no hemorrhaging in the ventricles, but consistent hemorrhaging in the parenchyma (middle; yellow arrowhead). None of the wild-type controls showed hemorrhaging in any section (left). Scale Bars = 100 μm.
Abnormal hemorrhage within the developing central nervous system of Clec1b−/− and Syk−/− mice from E12.5. (A) Both constitutive (Clec1b−/−; middle; n = 18 of 19) and platelet/megakaryocyte-specific (Clec1bfl/flPF4-Cre; right; n = 3 of 5) ablation of Clec1b results in hemorrhaging within the brain and developing spinal column (blue arrows). (B) Constitutive (Syk−/−; middle; n = 13 of 14) and platelet/megakaryocyte-specific (Sykfl/flPF4-Cre; n = 10 of 13) ablation of Syk presented with a similar hemorrhaging phenotype in similar regions as the Clec1b−/− embryos (blue arrows). None of the littermate wild-type controls (+/+; left; n ≥ 5) showed this phenotype. Scale bars = 1 mm. (C) H&E-stained sections from E12.5 brains show that hemorrhages in Clec1b−/− embryos were localized to at least 1 ventricle (middle; red arrowhead) and within the parenchyma (right; yellow arrowhead). (D) Sections from E12.5 Syk−/− embryos showed no hemorrhaging in the ventricles, but consistent hemorrhaging in the parenchyma (middle; yellow arrowhead). None of the wild-type controls showed hemorrhaging in any section (left). Scale Bars = 100 μm.
CLEC-2 is required in the hematopoietic lineage for normal lymphatic integrity
To investigate whether CLEC-2 was required in adult mice for normal lymphatic vessel integrity, we reconstituted the hematopoietic system of irradiated mice with wild-type or Clec1b−/− fetal liver cells as previously published.16 Seven weeks after reconstitution, radiation chimeras reconstituted with wild-type cells showed no abnormalities in the abdominal cavity, whereas chimeras reconstituted with CLEC-2–deficient cells showed blood in the mesenteric lymphatic vessels and in Peyer patches (supplemental Figure 3), suggesting that, as with Syk and SLP76,4 CLEC-2 was required in hematopoietic cells for integrity of lymphatic vessels. Interestingly, 12 weeks after reconstitution with Clec1b−/− fetal liver cells, 2 of 6 chimeras died, and the remaining 4 were killed because of deteriorating health. Bloody fluid was found in the chest cavity of these mice (4 of 6) on autopsy. In contrast, all chimeras reconstituted with wild-type fetal liver cells were healthy 12 weeks after reconstitution (n = 8). Because radiation is known to cause gastrointestinal syndrome,35 it is not readily possible to compare the severity of this phenotype with the phenotypes seen in adult mice produced by genetic approaches.
Megakaryocyte/platelet-specific ablation of CLEC-2 and Syk
To identify the cells in which loss of Syk resulted in the lymphatic phenotype, mice with a conditional loxP-flanked allele of Syk (Sykfl/fl) were crossed with mice expressing Cre recombinase under the control of tissue-specific promoters. Deletion of Syk in the whole hematopoietic system with the use of Vav-iCre resulted in the characteristic appearance of edema and blood-filled vessels in the skin of E14.5 embryos (Figure 4A). This phenotype appeared similar to that seen in Syk−/− embryos. However, unlike Syk−/− mice, there was little perinatal lethality, with most Sykfl/flVav-iCre mice surviving to adulthood (Table 2). Thus, Syk is required within the hematopoietic system for normal lymphatic development. In contrast, deletion of Syk in endothelial cells with the use of Tie1-Cre caused no detectable lymphatic phenotype during gestation (not shown) and no lethality (Table 2). To examine whether the reduced severity of the phenotype in Sykfl/flVav-iCre mice compared with Syk−/− mice was because of a role for Syk in endothelial cells secondary to that in hematopoietic cells, we deleted Syk in both lineages by crossing Sykfl/flVav-iCre mice with Tie1-Cre transgenic mice. In the resultant Sykfl/flVav-iCre Tie1-Cre mice the phenotype of blood-filled lymphatics at E14.5 was no more severe than that seen in Sykfl/flVav-iCre mice, and, mice survived to adulthood (Table 2). Taken together, these results show that normal lymphatic development requires Syk expression in hematopoietic cells but not in BECs.
Hematopoetic and platelet-specific deficiency of Syk and Clec1b result in defective lymphatic development. The phenotypes of (A) Sykfl/flVav-iCre, (B) Sykfl/flPF4-Cre, and (C) Clec1bfl/flPF4-Cre embryos at E14.5 are indistinguishable and include edematous swelling (asterisk), hemorrhages (blue arrow) and blood-filled lymphatics in the skin (black arrowhead). This phenotype is not seen in control littermates. The figures shown are representative of > 5 embryos of each genotype.
Hematopoetic and platelet-specific deficiency of Syk and Clec1b result in defective lymphatic development. The phenotypes of (A) Sykfl/flVav-iCre, (B) Sykfl/flPF4-Cre, and (C) Clec1bfl/flPF4-Cre embryos at E14.5 are indistinguishable and include edematous swelling (asterisk), hemorrhages (blue arrow) and blood-filled lymphatics in the skin (black arrowhead). This phenotype is not seen in control littermates. The figures shown are representative of > 5 embryos of each genotype.
Offspring resulting from Cre-transgenic matings
| Cre . | Total number of mice . | Number of Sykfl/flCre+ or Clec1bfl/flCre+ . | |
|---|---|---|---|
| Expected . | Found . | ||
| Syk | |||
| LysM | 139 | 34.8 | 51 |
| hCD2 | 93 | 18.9 | 18 |
| Vav | 207 | 54.5 | 35* |
| Tie1 | 155 | 37.4 | 28 |
| CD11c | 62 | 37.8 | 36 |
| PF4 | 108 | 32.5 | 17† |
| Tie1+Vav | 50 | 12.5 | 4 |
| Clec1b | |||
| PF4 | 81 | 29.8 | 26 |
| Cre . | Total number of mice . | Number of Sykfl/flCre+ or Clec1bfl/flCre+ . | |
|---|---|---|---|
| Expected . | Found . | ||
| Syk | |||
| LysM | 139 | 34.8 | 51 |
| hCD2 | 93 | 18.9 | 18 |
| Vav | 207 | 54.5 | 35* |
| Tie1 | 155 | 37.4 | 28 |
| CD11c | 62 | 37.8 | 36 |
| PF4 | 108 | 32.5 | 17† |
| Tie1+Vav | 50 | 12.5 | 4 |
| Clec1b | |||
| PF4 | 81 | 29.8 | 26 |
Lineage-specific deletion of Syk and Clec1b with the use of the Cre-recombinase lines indicated. The number of mice with homozygous deletion of Syk or Clec1b expected versus the actual number genotyped is shown.
Significant reduction in the number of mice as indicated by the χ2 test (P < .05).
Significant reduction in the number of mice as indicated by the χ2 test (P < .005).
To determine which hematopoietic lineage played a critical role in normal lymphatic–blood vessel separation, we crossed Sykfl/fl mice to hCD2-iCre, LysMCre, CD11c-Cre, and PF4-Cre transgenics, resulting in loss of Syk in B and T lymphocytes, in macrophages and neutrophils, in dendritic cells, or in megakaryocytes and platelets, respectively. No edema, blood-filled lymphatics, or perinatal lethality (Table 2) was seen in any of these crosses except in Sykfl/flPF4-Cre mice that had lost Syk expression in megakaryocytes and platelets (Figure 4B). Notably, Sykfl/flPF4-Cre mice showed a phenotype at E14.5 indistinguishable from that seen in Sykfl/flVav-iCre mice strongly suggesting that Syk was required in megakaryocytes and/or platelets for normal lymphatic development.
Extending this analysis to the lineage-specific requirement for CLEC-2, we generated a conditional loxP-flanked allele of Clec1b and crossed it to the PF4-Cre transgenic mouse. The resultant Clec1bfl/flPF4-Cre E14.5 embryos showed edema and blood-filled vessels in the skin, closely resembling the phenotype seen in Sykfl/flPF4-Cre embryos (Figure 4C). In addition, at E12.5, Clec1bfl/flPF4-Cre (3 of 5; Figure 3A) and Sykfl/flPF4-Cre (10 of 13; Figure 3B) embryos had hemorrhages within the developing brain similar to the constitutive loss of CLEC-2 and Syk, although generally they were fewer and smaller. Together these studies suggest that CLEC-2 and Syk are required in megakaryocytes and/or platelets during development by both the lymphatic and blood brain vasculatures.
Conditional deletion of Clec1b and Syk in megakaryocytes and platelets results in interconnected veins and lymphatics but does not affect perinatal lung function
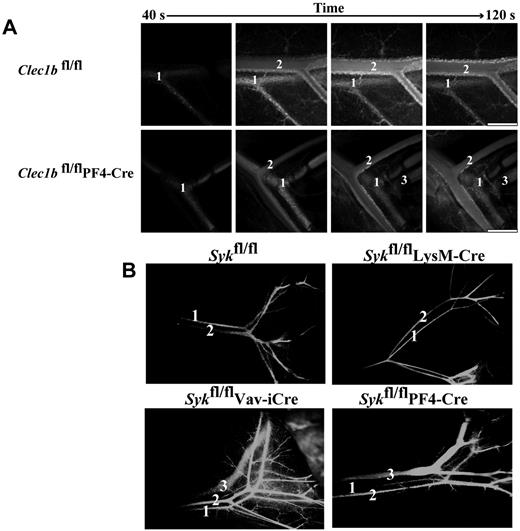
Next, we examined the phenotype of newborn and adult Clec1bfl/flPF4-Cre and Sykfl/flPF4-Cre mice. Both strains showed signs of defective lymphatics with blood visible in the mesenteric lymphatics and, in some cases, chylous fluid around the intestine (supplemental Figure 4; data not shown). Injection of FITC-dextran into the carotid artery of 8-week-old Clec1bfl/fl control mice led to sequential labeling of the mesenteric arteries and veins but not the lymphatic vessels, which run in parallel (Figure 5A; supplemental Video 1). In contrast, rapid labeling of the arteriolar, venous, and lymphatic systems could be readily seen in the Clec1bfl/flPF4-Cre mice, suggesting that there are direct interconnections between the blood and lymphatic vessels (Figure 5A). Labeling of the mesenteric lymphatics occurred ∼ 15 seconds after fluorescence was detected in the artery and several seconds after labeling of the veins (supplemental Video 2). The sudden appearance of the fluorescent dextran in the lymphatic vessels argues against a slow, remote leakage site. Similarly to Clec1bfl/fl mice, Sykfl/fl and Sykfl/flLysMCre mice show circulation of injected FITC-dextran only through the systemic blood vasculature (Figure 5B). In contrast, injection of FITC-dextran into the left ventricle of Sykfl/flVav-iCre and Sykfl/flPF4-Cre mice resulted in rapid labeling of the mesenteric lymphatic vessels (Figure 5B). Together, these results show that the loss of either CLEC-2 or Syk in the megakaryocyte/platelet lineage results in persistent inappropriate connections between the blood and lymphatic vasculatures of adult mice.
Disruption of Clec1b or Syk in the megakaryocyte/platelet lineage results in interconnection of the blood and lymphatic vasculatures. (A) FITC-dextran was injected into an anesthetized mouse via a carotid cannula after exteriorization of the abdominal mesentery. In both Clec1bfl/fl (top) and Clec1bfl/flPF4-Cre (bottom) FITC-dextran was initially visualized in the artery (1) followed shortly by the vein (2). In the Clec1bfl/flPF4-Cre mice FITC-dextran was visualized in the mesenteric lymphatic vasculature (3) shortly after the flowing through the vein. This phenomenon was never seen in the Clec1bfl/fl mice. Scale bar = 0.5 mm. This figure is representative of 3 experiments. (B) FITC-dextran was injected into the left ventricle of the heart of mice of the indicated genotypes, and vessels in the gut were visualized for FITC fluorescence 60 seconds later. FITC-dextran was only detected in the systemic blood circulation (1 and 2) of Sykfl/fl (top left) and Sykfl/flLysM-Cre mice (top right). In contrast, leakage of the FITC-dextran into the gut mesenteric lymphatic vasculature (3) was detected in Sykfl/flVav-iCre (bottom left) and Sykfl/flPF4-Cre (bottom right).
Disruption of Clec1b or Syk in the megakaryocyte/platelet lineage results in interconnection of the blood and lymphatic vasculatures. (A) FITC-dextran was injected into an anesthetized mouse via a carotid cannula after exteriorization of the abdominal mesentery. In both Clec1bfl/fl (top) and Clec1bfl/flPF4-Cre (bottom) FITC-dextran was initially visualized in the artery (1) followed shortly by the vein (2). In the Clec1bfl/flPF4-Cre mice FITC-dextran was visualized in the mesenteric lymphatic vasculature (3) shortly after the flowing through the vein. This phenomenon was never seen in the Clec1bfl/fl mice. Scale bar = 0.5 mm. This figure is representative of 3 experiments. (B) FITC-dextran was injected into the left ventricle of the heart of mice of the indicated genotypes, and vessels in the gut were visualized for FITC fluorescence 60 seconds later. FITC-dextran was only detected in the systemic blood circulation (1 and 2) of Sykfl/fl (top left) and Sykfl/flLysM-Cre mice (top right). In contrast, leakage of the FITC-dextran into the gut mesenteric lymphatic vasculature (3) was detected in Sykfl/flVav-iCre (bottom left) and Sykfl/flPF4-Cre (bottom right).
In contrast to the lymphatic defect, Clec1bfl/flPF4-Cre P0 pups and adults (6-8 weeks old) or Sykfl/flPF4-Cre P0 mice showed no visible abnormalities in the chest cavity. Lungs were fully expanded with no sign of hemorrhage or fluid collection. Histologic analyses showed patent conducting airways with normal alveoli (not shown). Because this phenotype was different from that observed in the constitutive deletion models, the efficiency of CLEC-2 and Syk ablation in adult platelets was analyzed by FACS and immunoblotting. CLEC-2 and Syk proteins were undetectable in platelets from adult Clec1bfl/flPF4-Cre and Sykfl/flPF4-Cre mice, respectively (supplemental Figure 5).
Platelet effects on lymphatic endothelial cell behavior are modulated by CLEC-2 and Syk
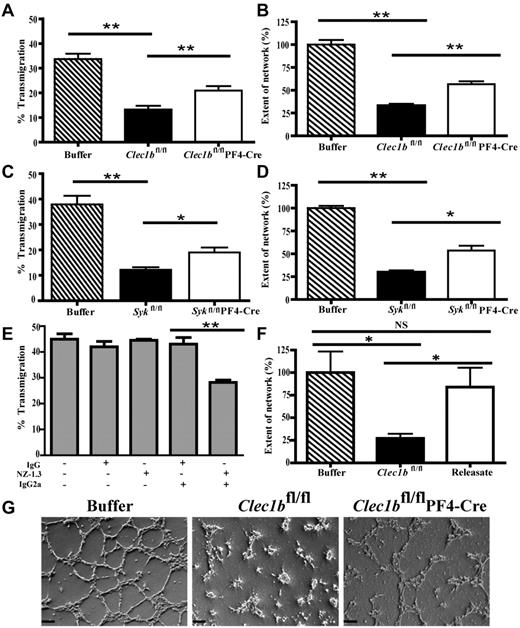
HLECs have been previously shown to induce aggregation of human platelets.17 Similarly, HLECs also cause aggregation of wild-type mouse platelets. This response was abrogated in Clec1b−/− mouse platelets, indicating that aggregation requires binding of podoplanin on the HLECs to CLEC-2 on platelets (supplemental Figure 6A). In contrast, the effect of platelets on LEC behavior is unknown. LEC migration plays a critical role in lymphangiogenesis. Therefore, to identify a potential mechanism by which platelets modulate lymphatic vasculature formation, we assessed the effect of platelets on LEC migration with the use of a transfilter assay.36 The presence of wild-type, Clec1bfl/flPF4-Cre, or Sykfl/flPF4-Cre platelets did not modify the total number of cells attached to the filters as measured at 24 hours (not shown), suggesting the presence of platelets did not affect cell survival or adhesion. The presence of platelets from Clec1bfl/fl or Sykfl/fl mice inhibited transmigration through the filter by > 60% relative to transmigration of HLECs not treated with platelets (Figure 6A,C). Platelets from Clec1bfl/flPF4-Cre and Sykfl/flPF4-Cre mice also decreased transmigration in comparison to untreated HLECs, but this effect was significantly weaker than that seen in the presence of platelets from Clec1bfl/fl or Sykfl/fl mice (Figure 6A,C). Application of platelet releasate did not affect LEC transmigration (supplemental Figure 6B). This suggests that platelets inhibit LEC migration in vitro by contact-dependent and CLEC-2/Syk–dependent and independent mechanisms.
Platelets can modulate lymphatic endothelial cell behavior. VEGF-C–driven migration of LECs in the absence or presence of washed platelets from Clec1bfl/fl or Clec1bfl/flPF4-Cre (A; n = 4) and Sykfl/fl or Sykfl/flPF4-Cre (C; n = 3) mice was assessed with the transfilter assay. The percentage of LECs that migrated through the filter was reduced significantly when platelets from Clec1bfl/fl or Sykfl/fl mice were applied to the LECs. Application of platelets from Clec1bfl/flPF4-Cre or Sykfl/flPF4-Cre mice significantly increased the amount of migration in comparison to the migration seen in the presence of platelets from Clec1bfl/fl or Sykfl/fl mice. Cross-linking of podoplanin (E) with the use of the Ab NZ-1.3 with a cross-linking secondary IgG2a resulted in a significant decrease in VEGF-C–mediated migration, whereas application of irrelevant IgG or the Ab without the cross-linking secondary showed no effect. Data represent mean values from ≥ 3 independent experiments performed in duplicate (mean ± SD; **P < .006, *P < .02). Network formation by LECs on Matrigel in the absence or presence of washed platelets from Clec1bfl/fl or Clec1bfl/flPF4-Cre (B) Sykfl/fl or Sykfl/flPF4-Cre (D) and Clec1bfl/fl platelet releasate (F) was assessed by seeding LECs in Matrigel-coated 12-well plates. The complexity of networks formed by LECs were reduced significantly when platelets from Clec1bfl/fl or Sykfl/fl mice were applied, whereas application of platelets from Clec1bfl/flPF4-Cre or Sykfl/flPF4-Cre mice only partially reduced network complexity, and platelet releasate showed no significant effects on network formation. Data represent mean values from 3 independent experiments performed in duplicate (mean ± SD; **P < .005, *P < .05). (G) Representative pictures of networks analyzed for the network-forming assay after application and incubation with either buffer (left) or platelets from Clec1bfl/fl (middle) or Clec1bfl/flPF4-Cre (right) mice. Scale bars = 100 μm.
Platelets can modulate lymphatic endothelial cell behavior. VEGF-C–driven migration of LECs in the absence or presence of washed platelets from Clec1bfl/fl or Clec1bfl/flPF4-Cre (A; n = 4) and Sykfl/fl or Sykfl/flPF4-Cre (C; n = 3) mice was assessed with the transfilter assay. The percentage of LECs that migrated through the filter was reduced significantly when platelets from Clec1bfl/fl or Sykfl/fl mice were applied to the LECs. Application of platelets from Clec1bfl/flPF4-Cre or Sykfl/flPF4-Cre mice significantly increased the amount of migration in comparison to the migration seen in the presence of platelets from Clec1bfl/fl or Sykfl/fl mice. Cross-linking of podoplanin (E) with the use of the Ab NZ-1.3 with a cross-linking secondary IgG2a resulted in a significant decrease in VEGF-C–mediated migration, whereas application of irrelevant IgG or the Ab without the cross-linking secondary showed no effect. Data represent mean values from ≥ 3 independent experiments performed in duplicate (mean ± SD; **P < .006, *P < .02). Network formation by LECs on Matrigel in the absence or presence of washed platelets from Clec1bfl/fl or Clec1bfl/flPF4-Cre (B) Sykfl/fl or Sykfl/flPF4-Cre (D) and Clec1bfl/fl platelet releasate (F) was assessed by seeding LECs in Matrigel-coated 12-well plates. The complexity of networks formed by LECs were reduced significantly when platelets from Clec1bfl/fl or Sykfl/fl mice were applied, whereas application of platelets from Clec1bfl/flPF4-Cre or Sykfl/flPF4-Cre mice only partially reduced network complexity, and platelet releasate showed no significant effects on network formation. Data represent mean values from 3 independent experiments performed in duplicate (mean ± SD; **P < .005, *P < .05). (G) Representative pictures of networks analyzed for the network-forming assay after application and incubation with either buffer (left) or platelets from Clec1bfl/fl (middle) or Clec1bfl/flPF4-Cre (right) mice. Scale bars = 100 μm.
The effects of Ab-mediated podoplanin cross-linking in HLECs were tested to determine whether platelet regulation of HLEC behavior relies on direct podoplanin cross-linking by CLEC-2. Treatment with anti–human podoplanin plus a secondary cross-linking Ab decreased VEGF-C–induced HLEC migration, whereas an irrelevant rat IgG or the primary Ab did not have any significant effect (Figure 6E). These data suggest that podoplanin cross-linking leads to the inhibition of HLEC migration, possibly because of altered constitutive podoplanin signaling.
To further characterize the modulatory role of platelets on HLEC behavior, we assessed the effect of platelets on HLEC network formation in vitro ≥ 3 hours after seeding the HLECs on Matrigel. The addition of Clec1bfl/fl or Sykfl/fl platelets significantly disrupted network formation by almost 70% (Figure 6B,D), whereas addition of Clec1bfl/fl platelet releasate had no effect (Figure 6F). This inhibitory effect was reduced ∼ 40% with the use of platelets from Clec1bfl/flPF4-Cre or Sykfl/flPF4-Cre mice (Figure 6B,D,G). Application of platelets to HUVEC cultures did not affect network formation (not shown).
These data show that direct contact of platelets affects the ability of HLECs to migrate, stabilize cell–cell interactions, and form networks in vitro in a CLEC-2/Syk–dependent manner.
Discussion
This study shows that loss of CLEC-2 and Syk in megakaryocytes and platelets results in similar defects in fetal lymphatic development, as does the loss of Syk within the whole hematopoietic lineage. In contrast, lineage-specific deletions of Syk from B and T lymphocytes, endothelial cells, macrophages and neutrophils, or dendritic cells do not result in defective lymphatics. These results suggest that CLEC-2 signaling via Syk in megakaryocytes/platelets is required for normal lymphangiogenesis which correspond to previous studies that used both Clec1b and SLP76 deletion models.15,22 The present study further shows that defects in lymphatic function in CLEC-2–deficient mice are associated with a decrease in the number of LECs relative to BECs and that platelets regulate LEC behavior via a contact-dependent mechanism that involves CLEC-2 and Syk. The altered migration and network formation capacity of LECs in the absence of platelet CLEC-2 or Syk may give rise to blood-filled lymphatics, possibly as a consequence of impaired separation of LECs from blood vessels or stabilization of mis-connections between the two vessels.
The lethality from constitutive deletion of CLEC-2 and Syk could be because of the functions of the two proteins in nonhematopoietic lineages, although this seems unlikely, given that CLEC-2 has not been detected outside the hematopoietic system. More probable, the milder phenotype, as evidenced by the viability of mice with PF4-Cre–mediated or Vav-Cre–mediated deletion, could be because of incomplete deletion of the genes. Although we were unable to detect expression of CLEC-2 or Syk in platelets from adult Clec1bfl/flPF4-Cre or Sykfl/flPF4-Cre, respectively, it is possible that a small fraction of cells still expressed protein which fell below the level of detection. Another explanation for the milder phenotype in the conditional mutants is that Clec1b and Syk undergo partial deletion in platelets formed by primitive rather than definitive hematopoiesis. Primitive hematopoiesis originates in the yolk sac at E6.5 with megakaryocyte/erythroid progenitors detected at E7.5 and yolk-sac–derived platelets appearing at E9.5.37-39 Megakaryocytes derived through primitive hematopoiesis have a lower ploidy level than those in fetal liver or BM, whereas their platelets are larger. Further, under culture conditions, platelets are generated more rapidly from primitive hematopoiesis relative to definitive hematopoiesis. Thus, although PF4 has been shown to be expressed during primitive hematopoiesis,37 it is possible that the more rapid formation of platelets enables residual levels of CLEC-2 and Syk to be expressed. The relative contribution of primitive and definitive hematopoiesis to lymphatic development is an important area for further investigation.
The present study reports that most of the constitutive CLEC-2 and Syk-deficient mice die shortly after birth. The lungs in these mice fail to inflate normally, and fluid is present in the larger airways. The few mice that survive for at least a few hours have marked difficulties in breathing. A similar pathology is seen in podoplanin-deficient mice in which most mice die within minutes of birth and have lungs lacking inflated airspaces.40 Because it appears that alveolar formation is disrupted in the constitutive CLEC-2 and Syk-deficient mice, podoplanin-expressing alveolar type-1 cells could be particularly affected by the absence of these proteins.
The disruption of lymphatic function is a potential cause of the fluid in the large airways of the Clec1b−/− and Syk−/− lungs and ultimately of their failure to inflate. During development, the airway epithelium secretes fluid into the lumen of the lung which influences its branching dynamics and structure.41 This liquid must be cleared at birth, and a large portion (∼ 40%) of the clearance is because of flow through lymphatics.42 The viability and apparently normal patency of the lungs in Clec1bfl/flPF4-Cre and Sykfl/flPF4-Cre mice could be because of a reduced severity of the lymphatic phenotype, which could allow lung drainage and therefore expansion to occur. However, it is also possible that megakaryocyte/platelet-expressed CLEC-2 and Syk are only partially involved or not involved in the lung defect and that, unlike the lymphatic defect, the lung disorder is the result of loss of these proteins in another cell type.
Preceding the appearance of blood-filled lymphatics CLEC-2– and Syk-deficient embryos showed hemorrhaging in the mid- and hind-brain of Clec1b−/− and Syk−/− embryos at E12.5. In Clec1b−/− embryos, blood was detected in at least 1 ventricle, whereas in Syk−/− embryos it was restricted to the parenchyma. These hemorrhagic foci persist throughout gestation. Tang et al have also reported hemorrhaging in the hind brain in a constitutive CLEC-2–deficient mouse.23 This phenotype is present in Clec1bfl/flPF4-Cre and Sykfl/flPF4-Cre mice, although it is less marked. This may reflect a role for CLEC-2 and Syk in other lineages or residual protein in a subset of platelets during early development as discussed earlier.
The hemorrhaging in the brain cannot be because of defective lymphatic function because this system is absent from the CNS. However, the choroid plexus, which is responsible for secretion of cerebrospinal fluid (CSF),43 expresses podoplanin during development.44 The podoplanin-expressing epithelial cells of the choroid plexus form a barrier between the blood and CSF which is distinct from the endothelial structure of the blood-brain barrier. Choroid plexus can be readily identified in histologic sections of embryos at day 12.5-13 in the fourth and lateral ventricles,45 which correspond with the sites of bleeding into the brain and shortly after the appearance of platelets. We, therefore, propose that platelet interaction with podoplanin-expressing cells of the choroid plexus may be important for the correct formation of the blood–CSF barrier.
There is considerable evidence of a role for podoplanin in regulating cell migration. Podoplanin up-regulation in cancer cells is associated with altered actin cytoskeleton reorganization and increased tumor cell migration and invasiveness.46 Similarly, in lung microvascular LECs, small interfering RNA–mediated podoplanin knockdown causes a dramatic reduction in directional migration47 and abrogated formation of capillary tubes on Matrigel.48 These effects appear to be independent of ligand engagement and may reflect constitutive signaling from podoplanin. Thus, changes in podoplanin-regulated migration of LECs as a consequence of interaction with CLEC-2– and Syk-dependent platelet activation could lead to altered lymphangiogenesis.
We show that platelets decrease VEGF-C–stimulated HLEC migration through a pathway that partially depends on CLEC-2 and Syk, raising the possibility that binding of CLEC-2 to podoplanin may inhibit migration. Moreover, the observed effects of platelets on formation of LEC networks on Matrigel suggest that activation of Syk after the binding of CLEC-2 to podoplanin destabilizes LEC–LEC interactions. These results are consistent with the observation that podoplanin-Fc, which interferes with endogenous ligand binding to podoplanin, also inhibits transmigration and network-forming ability of LECs.49 The partial effect of CLEC-2 and Syk deletion in these 2 assays suggests that additional platelet receptors or alternative mechanisms may also influence LEC function. An inhibitory action of CLEC-2 on podoplanin signaling however does not explain the similar in vivo phenotypes of CLEC-2 and Syk deficiency. It is therefore possible that activation of Syk by CLEC-2 is required in vivo to maintain binding of platelets to LECs and possibly the degree of clustering of podoplanin thereby influencing their modulatory effect. This hypothesis is supported by our data showing that cross-linking of podoplanin interferes with the migration of LECs and that disruption of network formation depends on the direct contact between platelets and LECs. This could be mediated through regulation of one or more platelet surface receptors, although this would appear to exclude platelet integrins because the phenotype has not been described in mice deficient in β1- or β3-integrins50,51 or in mice deficient in the global regulator of integrin function, Talin.
Clec1b−/− mice have a reduced LEC/BEC ratio, implying a reduction in LEC number because BECs have not been reported to be affected by the deletion of CLEC-2, Syk, or SLP76. This reduction opens up several questions about the nature of the lymphatic defect in these mice, because platelets have not been shown to alter proliferation, survival, or differentiation of LECs, at least in vitro.22 These results, in conjunction with the results of the migration and network assays, suggest a critical role for CLEC-2 in the establishment of functional lymphatic vessels.
In summary, with the use of several unique lineage-specific deletion mouse models, this study shows the critical role of platelet CLEC-2 and Syk in lymphangiogenesis and in the development of the brain vasculature and found that platelets directly influence LEC migration and formation of junctions through a CLEC-2– and Syk-dependent process.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Milan Fernando, Beata Grygielska, Phil Stone, and Hannah Jeffery for excellent technical assistance.
This work was supported by the Wellcome Trust (ref: 088410). L.N.N. holds a postdoctoral fellowship from the Spanish Ministry of Education (EX2009-0242). E.S. and V.L.J.T. are supported by the Medical Research Council UK (Program no. U117527252). S.P.W. holds a British Heart Foundation Chair (CH/03/003).
Authorship
Contribution: B.A.F., E.S., L.N.-N., C.B., F.B., C.E.H., S.A.L., and K.L.L. performed experiments; A.Y.P., D.M.-S., S.S., G.B.N., N.S., and C.R.e.S. provided reagents and mouse models; B.A.F., V.L.J.T., and S.P.W. wrote the manuscript with critical editing provided by all of the authors; and V.L.J.T. and S.P.W. designed experiments and oversaw the research program.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for S.S. is Takeda Cambridge, Cambridge, United Kingdom.
Correspondence: Victor L. J. Tybulewicz, MRC National Institute for Medical Research, London NW7 1AA, United Kingdom; e-mail: vtybule@nimr.mrc.ac.uk; and Steve P. Watson, Centre for Cardiovascular Sciences, Institute for Biomedical Research, College of Medical and Dental Sciences, University of Birmingham, Birmingham B15 2TT, United Kingdom; e-mail: s.p.watson@bham.ac.uk.
References
Author notes
B.A.F. and E.S. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal