Abstract
Most human transfusion recipients fail to make detectable alloantibodies to foreign RBC antigens (“nonresponders”). Herein, we use a murine model to test the hypothesis that nonresponders may be immunologically tolerant. FVB mice transfused with RBCs expressing transgenic human glycophorin A (hGPA) antigen in the absence of inflammation produced undetectable levels of anti-hGPA immunoglobulins, unlike those transfused in the presence of polyinosinic:polycytidylic acid–induced inflammation. Mice in the nonresponder group failed to produce anti-hGPA after subsequent transfusions in the presence of polyinosinic:polycytidylic acid, whereas anti-hGPA levels increased in the responder group. This tolerance was antigen specific, because nonresponders to hGPA produced alloantibodies to RBCs that expressed a different transgenic antigen. This tolerance was not an idiosyncrasy of the hGPA antigen nor of the recipient strain, because B10.BR mice transfused with membrane-bound hen egg lysozyme antigen–transgenic RBCs also demonstrated induced nonresponsiveness. These data demonstrate that RBCs transfused in the absence of inflammation can induce tolerance.
Introduction
Although a transfused RBC unit typically contains many mismatched antigens between donor and recipient, a small minority of transfusion recipients (3%-6%) make detectable anti-RBC alloantibodies.1,2 Certain requirements must be met for alloimmunization to occur, including appropriate presentation of the foreign antigen by the recipient's antigen-presenting cells3 ; however, variable alloantibody response rates (20%-80%) are observed even for antigens such as Rh(D), thought to be nearly universally capable of presentation by the recipient's immune system.4-6
As an immunology paradigm, presentation of the same antigen under one set of conditions may lead to tolerance, whereas presentation under a different set of conditions (such as in the presence of a danger signal) may lead to immunity.7 In the setting of an RBC transfusion, a danger signal may come from either the transfused product itself (eg, cytokines, white blood cells, damaged RBCs, or bacteria) or from recipient factors (eg, underlying disease, infection, or genetic status). Canonically, these factors influence outcomes through activation or repression of innate immune responses that regulate subsequent adaptive immunity (eg, costimulatory or coinhibitory responses).
Humans may be immunologic “responders” or “nonresponders” to antigens on transfused RBCs.8,9 Responders are more likely to produce RBC antibodies on future transfusion exposure, which is only weakly dependent on transfusion number and may be genetically determined. Although patients with certain disease states (eg, sickle cell disease) have higher baseline rates of RBC alloimmunization,10-14 responder and nonresponder subgroups are thought to exist within these populations as well. The mechanisms by which nonresponders fail to make RBC alloantibodies, however, are poorly understood.
We and others have shown that murine recipients transfused in their baseline state produce undetectable or low levels of RBC alloantibodies, whereas those transfused in the presence of inflammation have higher rates and magnitude of alloimmunization.15-18 We now hypothesize that transfusion in the absence of inflammation leads to tolerance as opposed to simple nonresponsiveness and test this hypothesis using model systems in which transgenic human glycophorin A antigen (hGPA)19 or membrane-bound hen egg lysozyme antigen (mHEL)20 are present on transfused RBCs.
Methods
Mice
FVB, C57BL/6, and B10.BR mice were purchased from The Jackson Laboratory. HOD (RBC-specific expression of hen egg lysozyme, ovalbumin, and human Duffy b),21 hGPA (RBC-specific expression of human glycophorin A, generously provided by the New York Blood Center),19 and mHEL (ubiquitous expression of membrane-bound hen egg lysozyme)20 mice were bred by the Emory University Division of Animal Resources. Transfusion-recipient mice were 8-16 weeks of age, and all protocols were approved by the Emory University Institutional Animal Care and Use Committee.
Blood collection and transfusion
Blood was collected into acid-citrate-dextrose and washed with PBS. Blood from mHEL mice was leukoreduced, given the ubiquitous expression of mHEL on all cell types,15 whereas blood from the other transgenic blood donors used (HOD and hGPA) was not leukoreduced, given the presumed RBC-specific expression of these antigens.19,21 Packed RBCs (100 μL) were transfused via lateral tail vein15 ; some recipients were pretreated with 100 μg of polyinosinic:polycytidylic acid [poly(I:C); Amersham] 4 hours before transfusion. B10.BR recipients were used for mHEL transfusions because of the inability of C57BL/6 mice to process the hen egg lysozyme (HEL) antigen into an I-Ab binding determinant (a portion of HEL cannot be presented by I-Ab class II MHC of C57BL/6 mice)22 ; FVB recipients were used for hGPA, FVB, or HOD transfusions. Some recipients were immunized subcutaneously in the flank with HEL or ovalbumin protein (Sigma-Aldrich) emulsified in complete Freund adjuvant (concentration of 2 μg/μL; total protein injected per mouse 100 μg).23 Supplemental Figures 1 and 2 show experimental design schematics (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Antibody detection
Two weeks after transfusion, anti-hGPA, anti-HOD, or anti-HEL antibodies were measured in recipients by flow cross-matching with C57BL/6, HOD, mHEL, hGPA, or FVB RBCs as described previously.15 Adjusted mean fluorescence intensity was calculated by subtracting the background signal of sera cross-matched with control RBCs from that of the desired targets. In a subset of experiments in which the anti-HEL response was too low to be detected by flow cross-matching, an anti-HEL–specific ELISA was performed15 ; HEL-specific ELISAs are approximately 100 times more sensitive than flow cytometric cross-matching with mHEL RBCs, with flow being 30 times more sensitive than agglutination-based assays.15
Statistical analysis
Statistical analysis was performed with GraphPad Prism software. One-way ANOVAs with Bonferroni posttest or Mann-Whitney U tests were performed, with a statistically significant value defined as P < .05.
Results and discussion
Response to transfused hGPA or mHEL RBCs
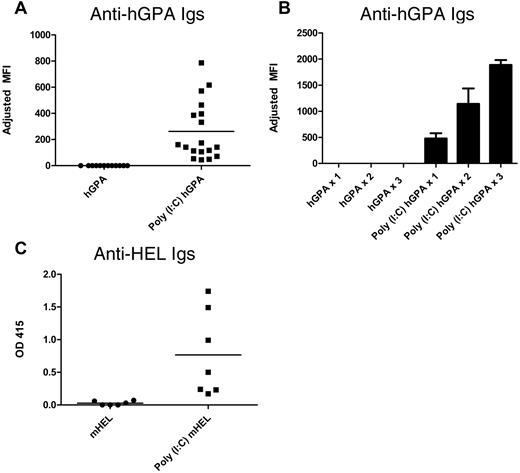
FVB (H2q) recipients were transfused with the equivalent of 1 “unit” of hGPA RBCs (100 μL of packed RBCs) in the presence or absence of recipient treatment with poly(I:C). Sera collected 2 weeks after transfusion were cross-matched with hGPA or wild-type FVB RBCs, with the difference being the adjusted mean fluorescence intensity. In 8 of 8 experiments (80 mice total), no recipient of hGPA RBCs transfused in the absence of inflammation produced detectable anti-hGPA antibodies (“nonresponders”; Figure 1A). This was not because of an inability of FVB mice to make anti-hGPA antibodies, because 100% of FVB recipients transfused with hGPA RBCs in the presence of poly(I:C) developed a detectable anti-hGPA response (Figure 1A). Nonresponders to hGPA RBCs failed to make anti-hGPA after 2 additional hGPA RBC transfusions given 3 weeks apart, whereas mice in the poly(I:C) responder group had higher anti-hGPA responses after being boosted with poly(I:C) and hGPA RBCs (Figure 1B). To determine whether the nonresponsiveness was unique to FVB recipients, C57BL/6 recipients were transfused a total of 5 times (3 weeks apart) with hGPA RBCs in the absence of poly(I:C). Like FVB recipients, C57BL/6 recipients transfused in the absence of poly(I:C) failed to produce detectable anti-hGPA responses, although they did have detectable responses to donor MHC (H2q; data not shown).
hGPA or mHEL RBCs are nonimmunogenic (or weakly immunogenic) when transfused into murine recipients in their baseline states. (A) Anti-hGPA response by flow cross-matching in FVB recipients transfused once with 1 “unit” of hGPA RBCs (100 μL of packed RBCs) in the presence or absence of poly(I:C); compilation of 3 representative experiments, 30 recipients total. Adjusted mean fluorescence intensity (MFI) was 0 for the hGPA group and 262 for the poly(I:C) hGPA group (P < .0001). (B) Anti-hGPA response by flow cross-matching in FVB recipients (10 total) after each of 3 hGPA transfusions given 3 weeks apart in the presence or absence of poly(I:C). Mean adjusted MFI for hGPA group after 1, 2, and 3 transfusions: 0. Mean adjusted MFI for poly(I:C) hGPA group after 1, 2, and 3 transfusions: 477.2, 1141, and 1887, respectively. SD depicted by error bars. (C) Anti-HEL response by ELISA in B10.BR recipients transfused once with 1 “unit” of leukoreduced mHEL RBCs in the presence or absence of poly(I:C). Representative experiment with 13 mice total; mean optical density 0.03 for the mHEL group and 0.77 for the poly(I:C) mHEL group (P < .005). This anti-HEL response in poly(I:C) mHEL–immunized recipients was not boosted by additional poly(I:C) mHEL transfusions. OD 415 indicates optical density at 415 nm.
hGPA or mHEL RBCs are nonimmunogenic (or weakly immunogenic) when transfused into murine recipients in their baseline states. (A) Anti-hGPA response by flow cross-matching in FVB recipients transfused once with 1 “unit” of hGPA RBCs (100 μL of packed RBCs) in the presence or absence of poly(I:C); compilation of 3 representative experiments, 30 recipients total. Adjusted mean fluorescence intensity (MFI) was 0 for the hGPA group and 262 for the poly(I:C) hGPA group (P < .0001). (B) Anti-hGPA response by flow cross-matching in FVB recipients (10 total) after each of 3 hGPA transfusions given 3 weeks apart in the presence or absence of poly(I:C). Mean adjusted MFI for hGPA group after 1, 2, and 3 transfusions: 0. Mean adjusted MFI for poly(I:C) hGPA group after 1, 2, and 3 transfusions: 477.2, 1141, and 1887, respectively. SD depicted by error bars. (C) Anti-HEL response by ELISA in B10.BR recipients transfused once with 1 “unit” of leukoreduced mHEL RBCs in the presence or absence of poly(I:C). Representative experiment with 13 mice total; mean optical density 0.03 for the mHEL group and 0.77 for the poly(I:C) mHEL group (P < .005). This anti-HEL response in poly(I:C) mHEL–immunized recipients was not boosted by additional poly(I:C) mHEL transfusions. OD 415 indicates optical density at 415 nm.
To determine whether these observations were an idiosyncrasy of the hGPA antigen, B10.BR recipients were transfused with leukoreduced mHEL RBCs. Similar to what we have reported previously,15 low levels of or no anti-HEL Igs were detected by sensitive ELISA after a single mHEL transfusion in the absence of poly(I:C), whereas an enhanced response was observed in the presence of poly(I:C) (Figure 1C).
Alloantibody levels in nonresponder or low-responder animals after subsequent transfusions
To determine whether nonresponder animals were capable of producing an anti-hGPA response to a subsequent transfusion, FVB nonresponder recipients were transfused 6 weeks after the first hGPA transfusion with poly(I:C) hGPA; in this setting, no mouse produced a detectable anti-hGPA response (Figure 2A). This was not because hGPA was nonimmunogenic, because naive animals transfused with poly(I:C) hGPA all produced detectable anti-hGPA. It was also not because of general immune suppression induced by prior RBC exposure, because recipients previously transfused with syngeneic FVB RBCs had robust anti-hGPA responses (Figure 2A). The lack of an anti-hGPA response appears to be allospecific, because 100% of mice initially nonresponsive to hGPA RBCs were capable of making antibodies (anti-HOD) to a third-party antigen on subsequent transfusion with poly(I:C) HOD or poly(I:C) HOD × hGPA F1 RBCs (Figure 2B).
Antigen-specific “nonresponsiveness” persists after subsequent RBC transfusion in the presence of inflammation. (A) Six weeks after hGPA or wild-type FVB RBC transfusion, recipients were challenged with hGPA RBCs in the presence of poly(I:C), with anti-hGPA levels assessed by flow cross-matching after the final transfusion. Compilation of 3 experiments, 29 recipients total. Adjusted mean fluorescence intensity (MFI) was 119 after poly(I:C) hGPA transfusion in the group initially not transfused, 103 in the group initially transfused with hGPA RBCs, and 1 in the group initially transfused with FVB RBCs. P < .0001 between the group initially not transfused and the hGPA group; P = .6 between the hGPA and FVB transfused groups. No anti-hGPA was produced in any group 2 weeks after the initial transfusion. (B) FVB recipients were either not transfused or transfused with hGPA RBCs, then transfused again 6 weeks later with HOD or HOD × hGPA F1 RBCs in the presence of poly(I:C), with anti-HOD assessed by flow cross-matching after the final transfusion. Compilation of 4 experiments, 40 recipients total. Mean adjusted MFI was 56 after poly(I:C) HOD or HOD × hGPA F1 in the group initially not transfused compared with 44 in the group initially transfused with hGPA RBCs (P = .65). No anti-hGPA or anti-HOD was detected in either group after the initial hGPA transfusion, and no anti-hGPA was detected after the final transfusion. (C) B10.BR recipients were transfused with mHEL or control C57BL/6 RBCs and then boosted 6 weeks later with mHEL RBCs in the presence of poly(I:C), with anti-HEL assessed by ELISA 2 weeks after the first and last transfusions. Compilation of 2 experiments with 18 recipients. Mean optical density (OD) was 0.45 before and 0.33 after poly(I:C) mHEL transfusion in the group initially transfused with mHEL RBCs; OD was 0.1 before and 1.1 after poly(I:C) mHEL transfusion in the group initially transfused with C57BL/6 RBCs (P = .004 between groups after the last transfusion). (D) B10.BR recipients were transfused with mHEL or control C57BL/6 RBCs and then injected subcutaneously 6 weeks later with HEL protein emulsified in complete Freund's adjuvant (CFA), with anti-HEL assessed 2 weeks after this immunization by flow cross-matching. Compilation of 2 experiments, 21 recipients total. Adjusted MFI was 3 after HEL/CFA treatment in the group initially transfused with mHEL RBCs compared with 38 in the group initially transfused with C57BL/6 RBCs (P = .0006).
Antigen-specific “nonresponsiveness” persists after subsequent RBC transfusion in the presence of inflammation. (A) Six weeks after hGPA or wild-type FVB RBC transfusion, recipients were challenged with hGPA RBCs in the presence of poly(I:C), with anti-hGPA levels assessed by flow cross-matching after the final transfusion. Compilation of 3 experiments, 29 recipients total. Adjusted mean fluorescence intensity (MFI) was 119 after poly(I:C) hGPA transfusion in the group initially not transfused, 103 in the group initially transfused with hGPA RBCs, and 1 in the group initially transfused with FVB RBCs. P < .0001 between the group initially not transfused and the hGPA group; P = .6 between the hGPA and FVB transfused groups. No anti-hGPA was produced in any group 2 weeks after the initial transfusion. (B) FVB recipients were either not transfused or transfused with hGPA RBCs, then transfused again 6 weeks later with HOD or HOD × hGPA F1 RBCs in the presence of poly(I:C), with anti-HOD assessed by flow cross-matching after the final transfusion. Compilation of 4 experiments, 40 recipients total. Mean adjusted MFI was 56 after poly(I:C) HOD or HOD × hGPA F1 in the group initially not transfused compared with 44 in the group initially transfused with hGPA RBCs (P = .65). No anti-hGPA or anti-HOD was detected in either group after the initial hGPA transfusion, and no anti-hGPA was detected after the final transfusion. (C) B10.BR recipients were transfused with mHEL or control C57BL/6 RBCs and then boosted 6 weeks later with mHEL RBCs in the presence of poly(I:C), with anti-HEL assessed by ELISA 2 weeks after the first and last transfusions. Compilation of 2 experiments with 18 recipients. Mean optical density (OD) was 0.45 before and 0.33 after poly(I:C) mHEL transfusion in the group initially transfused with mHEL RBCs; OD was 0.1 before and 1.1 after poly(I:C) mHEL transfusion in the group initially transfused with C57BL/6 RBCs (P = .004 between groups after the last transfusion). (D) B10.BR recipients were transfused with mHEL or control C57BL/6 RBCs and then injected subcutaneously 6 weeks later with HEL protein emulsified in complete Freund's adjuvant (CFA), with anti-HEL assessed 2 weeks after this immunization by flow cross-matching. Compilation of 2 experiments, 21 recipients total. Adjusted MFI was 3 after HEL/CFA treatment in the group initially transfused with mHEL RBCs compared with 38 in the group initially transfused with C57BL/6 RBCs (P = .0006).
To explore nonresponsiveness in a different antigen system, B10.BR animals transfused with mHEL or C57BL/6 RBCs were transfused 6 weeks later with poly(I:C) mHEL or injected subcutaneously with HEL protein emulsified in complete Freund adjuvant. In a compilation of 4 experiments, no increase in anti-HEL beyond that observed after the initial mHEL transfusion was observed in recipients transfused first with mHEL RBCs and then with poly(I:C) mHEL, and these responses were low enough to be detected only by sensitive ELISA (Figure 2C); prior mHEL transfusion also blunted but did not completely eliminate the response to subcutaneous HEL protein emulsified in complete Freund adjuvant (Figure 2D). The HEL nonresponsiveness was antigen specific, because similar responses to immunization with a third-party antigen (ovalbumin) emulsified in complete Freund adjuvant were seen in all experimental groups (data not shown).
In summary, the data contained herein demonstrate that responder/nonresponder status can be determined by recipient inflammatory and innate immune activation status. Although stochastic RBC alloimmunization modeling data in humans suggest that responder/nonresponder status is a function of the recipient's genetic repertoire,8 this does not exclude a role for environmental factors in regulating immune responses within patient populations with the genetic ability to respond to a given antigen. Future directions for research include a thorough analysis of immune mechanisms responsible for the observed nonresponsiveness, an investigation of the sustainability of the nonresponsive state in the absence of ongoing antigen exposure, and an exploration of blood group antigen characteristics that may themselves play a role in determining whether tolerance can be induced under certain environmental conditions. A more complete understanding of these factors may lay the groundwork for the development of novel strategies for tolerance induction to RBC antigens in patients at risk of RBC alloimmunization.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by a grant from the National Institutes of Health (HL069769) to J.E.H.
National Institutes of Health
Authorship
Contribution: N.H.S. and J.E.H. performed the research; E.A.H., S.L.S., J.C.Z., and J.E.H. designed the research; and all authors analyzed the data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jeanne Hendrickson, MD, 101 Woodruff Circle, 7105B Woodruff Memorial Bldg, Atlanta, GA 30322; e-mail: jeanne.hendrickson@choa.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal