Abstract
Loss of major histocompatibility complex class II (MHC II) expression is associated with poor patient outcome in diffuse large B-cell lymphoma (DLBCL). As MHC II molecules are lost with plasmacytic differentiation in normal cells, we asked whether MHC II loss in DLBCL is associated with an altered differentiation state. We used gene expression profiling, quantum dots, and immunohistochemistry to study the relationship between MHC II and plasma cell markers in DLBCL and plasmablastic lymphoma (PBL). Results demonstrate that MHC II(−) DLBCL immunophenotypically overlap with PBL and demonstrate an inverse correlation between MHC II and plasma cell markers MUM1, PRDM1/Blimp1, and XBP1s. In addition, MHC II expression is significantly higher in germinal center-DLBCL than activated B cell-DLBCL. A minor subset of cases with an unusual pattern of mislocalized punctate MHC II staining and intermediate levels of mRNA is also described. Finally, we show that PBL is negative for MHC II. The results imply a spectrum of MHC II expression that is more frequently diminished in tumors derived from B cells at the later stages of differentiation (with complete loss in PBL). Our observations provide a possible unifying concept that may contribute to the poor outcome reported in all MHC II(−) B-cell tumors.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is an aggressive neoplasm of B cells, which accounts for almost 40% of all non-Hodgkin lymphoma cases.1-4 It is a disease marked by heterogeneity in clinical presentation, morphology, and underlying biology. As such, patient outcome is variable; 5-year survival is approximately 50%.4 A number of distinct molecular subtypes have been identified based on morphologic studies, immunophenotyping, genetics, and gene expression profiling (GEP). These data have led to the concept that DLBCL originates from at least 2 normal cellular counterparts: a peripheral B cell of the germinal center (GCB-DLBCL) or a post–germinal center (activated) B cell (ABC-DLBCL), with the remaining cases being difficult to classify (unclassifiable-DLBCL).1,5,6 Primary mediastinal B-cell lymphoma (PMBCL) has also been shown to have unique features that separate it from DLBCL into a distinct disease entity.7
Plasmablastic lymphoma (PBL) is another B-cell lymphoma characterized by a diffuse proliferation of large B cells with a plasma cell immunophenotype and very poor prognosis.1 It was originally described in the oral cavity but is found in other sites, predominantly extranodal mucosal sites. It is an uncommon disease, is often associated with immunodeficient states, and is usually Epstein-Barr virus-positive. Its postulated normal counterpart is a plasmablast, a blastic, proliferating B cell with a plasma cell immunophenotype.1 PBL was originally described as a variant of DLBCL8 but has since been classified as a distinct clinical entity.1
Major histocompatibility complex (MHC) molecules are transmembrane glycoproteins that present peptides for antigen recognition and are important for the adaptive immune response. MHC class II (MHC II) proteins are limited to expression on the surface of antigen-presenting cells, including B cells, and are critical for the protective immune response to pathogens and tumors. MHC II molecules are expressed by mature B cells but are lost with plasmacytic differentiation. In humans, MHC molecules are referred to as human leukocyte antigens (HLAs); HLA-DR is the most highly expressed isoform of the family.9,10
Because MHC II proteins are expressed on normal B cells, DLBCLs are generally expected to express MHC II as well. However, variation in MHC II was one of the major prognostic molecular signatures found in GEP of DLBCL, which was independent of the ABC- or GCB-cell of origin.6 Loss of MHC II protein expression has been documented in a variety of B-cell neoplasms and is associated with an aggressive clinical course.11-17 MHC II loss has been associated with poor survival, independent of clinical prognostic variables, in DLBCLs treated with various regimens,18,19 including MACOP-B therapy,20 CHOP therapy,6,21-23 risk-adapted therapy,24 and R-CHOP therapy,25 as well as in CHOP-treated PMBCLs.26,27 In addition to lost expression, aberrant cytoplasmic protein expression of MHC II has been documented in Hodgkin lymphoma, and when grouped with true MHC II(−) cases, correlated with reduced survival.28 The relationship between loss of MHC II and decreased survival is probably the result of decreased immunosurveillance, as a number of studies have demonstrated that loss of MHC II (and class I) on malignant cells is associated with a poor host tumor-infiltrating T-cell response.21,22,27,29-31
Although the mechanism of MHC II loss remains unknown, our investigations to date suggest that an altered transcriptional program is involved.32-35 As B-cell differentiation is controlled largely via transcription and decreased MHC II expression is one of the normal changes seen as B cells differentiate into mature, antibody-secreting plasma cells, we hypothesized that MHC II loss in DLBCL may be indicative of a more advanced differentiation state.
In this study, we used GEP data, quantum dot immunohistochemistry (IHC), and standard IHC to investigate MHC II expression in the context of B-cell and plasma cell markers in cases of DLBCL and PBL. Our overall hypothesis was that cases of DLBCL that have lost MHC II would express more plasma cell markers than MHC II(+) DLBCL. GEP was used to study the relationship between expression of MHC II and plasma cell markers MUM1, PRDM1/Blimp1, and XBP1s at the mRNA level, and to place MHC II loss in the context of DLBCL cell-of-origin subtypes. We used quantum dot IHC to score the expression of MHC II (HLA-DR), B-cell marker CD20, and plasma cell marker PRDM1/Blimp1 in cases of DLBCL, using PBL cases for comparison. We further explored cases of DLBCL using standard IHC for B-cell MHC II, HLA-DR, CD20, and plasma cell (CD138, MUM1, PRDM1/Blimp1, XBP1s) markers. We compared expression of these markers between cases of DLBCL that do or do not express MHC II, as previously defined by GEP.6
Methods
Approval for this study was obtained from the University of Arizona Institutional Review Board in accordance with the Declaration of Helsinki.
GEP data analysis
We used available microarray data from the Affymetrix Human Genome U133A and U133B arrays (Affymetrix) for 203 cases of DLBCL. These data were previously generated by our research consortium and consisted of gene expression data from frozen tissues on a subset of 203 cases of DLBCL from the 240 cases in Rosenwald et al.6 Affymetrix expression values are in arbitrary units and have been normalized to a trimmed mean signal of 500 using MAS Version 5.0 software (Affymetrix). Array elements were verified as described previously.21,32 All of the microarray elements for each gene that passed our criteria were averaged to give a single set of expression values for each gene, which were used in subsequent analysis. MHC II expression was defined as the average of the expression of all the classical and nonclassical MHC II genes present in the dataset: HLA-DRA, -DRB, -DPA, -DPB, -DQA, -DQB, -DMA, -DMB, -DOA, -DOB, and Ii. As previously published, there is a high degree of correlation between expression of the different classical and nonclassical MHC II genes and between individual molecules and the average of the set.21,32 We therefore used the average expression of all of the MHC II genes with the assumption that this would be most representative of overall tumor biology.
Tissues
All cases for slide-based studies were formalin-fixed, paraffin-embedded tissue sections. For quantum dot studies, whole tissue sections of 14 cases of DLBCL were obtained from University Medical Center, Tucson, AZ. These cases represented a subset of the cohort described previously, for which MHC II levels had been determined by GEP.6,21 These 14 cases were all considered MHC II(+) by GEP but were separated into MHC II(+) (8 cases) and MHC II-punctate (6 cases), as determined by IHC (as described further in “Quantum dot fluorescent IHC”). An additional 14 cases of DLBCL were obtained from Memorial Sloan-Kettering and were separated into MHC II(−) (7 cases) and MHC II-punctate (7 cases), as determined by IHC. Fifteen cases of PBL, as defined by the WHO (2001),36 were also obtained from Memorial Sloan-Kettering; pathology records were searched with an approved institutional review board waiver, as well as a log from a previously published series on PBL.37 For IHC studies, 40 cases of DLBCL were obtained from University Medical Center, Tucson, AZ. These cases represented a subset of the cohort described previously, for which MHC II levels had been determined by GEP.6,21 The 40 cases were arranged on a tissue microarray, with three 0.6-mm punches per case, including 2 cases of MHC II less than 10%, 6 cases of 10% to 25%, 7 cases of 25% to 50%, and 25 cases of more than 50%. Thus, cases with limited MHC II expression are under-represented on this array.
The DLBCL cases from University Medical Center, Tucson, AZ used in the current study are the same cases that were analyzed in our previous publications investigating the mechanism of lost MHC II expression in DLBCL. Previously published information has included expert pathology review, GEP analysis, metaphase CGH, positional expressional profiling, mutational analysis of both CIITA and RFX (the main transactivator and a key transcription factor of MHC II, respectively), and CIITA promoter methylation analysis. These studies show that such genetic or structural causes for decreased MHC II expression are not important mechanisms in these cases.21,32-34
Quantum dot fluorescent IHC
Quantum dot IHC.
To colocalize MHC II with B-cell or plasma cell markers within the same cell, we performed multiplexed IHC on a DiscoveryXT automated stainer (Ventana Medical Systems) using the following primary antibodies: mouse anti-CD20 (Ventana Medical Systems; neat), rabbit anti–HLA-DRa (Epitomics Inc; 1:80), mouse anti-PRDM1/Blimp1 (CNIO, neat), and 4,6-diamidino-2-phenylindole (DAPI) nuclear stain (Ventana Medical Systems). Primary antibodies were applied sequentially (PRDM1/Blimp1, HLA-DRa, CD20), followed by appropriate biotinylated anti–mouse or anti–rabbit secondary antibodies (Ventana Medical Systems). Intermediate blocking and washing steps were performed with an Avidin/Biotin Blocker (Ventana Medical Systems), and detection was accomplished by applying streptavidin-conjugated quantum dots: Qdot-625 (red), Qdot-585 (yellow), or Qdot-525 (green; Invitrogen), respectively.
Scoring.
Slides were viewed at 10×, 20×, and 40× magnification (Plan Fluor; Nikon Instruments) using an Olympus BX-61 microscope system (Olympus America). Quantum dots were excited using an X-Cite Exacte light-guide coupled and stabilized metal halide excitation source (Lumen Dynamics) at 2.46-W broadband output at the light guide end. A long-pass filter with a 410-nm cutoff was used to examine overall morphology and to select a representative area with a high tumor percentage for evaluation. Qdot-525 (CD20) expression was scored using the long-pass filter. Individual emission filters (Omega Optical) with 30-nm bandwidth centered about quantum dot wavelength were used for visualization and scoring of Qdot-585 (HLA-DR) or Qdot-625 (PRDM1/Blimp1). Tonsil tissue was used as a control to facilitate evaluation of intensity and pattern of staining in normal cells, with particular attention to CD20 in B cells, HLA-DR in macrophages, and PRDM1/Blimp1 in plasma cells. CD20 was assessed for membranous staining; HLA-DR was assessed for membranous staining, with notation made of nonmembrane/punctate cytoplasmic staining; and PRDM1/Blimp1 was assessed for nuclear staining. Scoring was performed via direct inspection by eye and detailed the percentage of cells that fell into intensity brackets of 0 to 3+ (0 indicates no staining; 1+, faint partial staining; 2+, complete or partial moderate staining; and 3+, complete strong staining). Protein expression scores using quantum dots were calculated by summing each intensity value times percent cells in that bracket (ie, score = (percent of (0) cells × 0) + (percent of (1+) cells × 1) + (percent of (2+) cells × 2) + (percent of (3+) cells × 3)). Thus, calculated protein expression scores using quantum dots had a possible range of expression of 0 to 300.
Chromogenic IHC
IHC.
IHC was performed on a Ventana Benchmark XT automated slide preparation system (Ventana Medical Systems). The iView DAB detection kit (Ventana Medical Systems) was used for antigen detection with the following primary antibodies: mouse anti–HLA-DR (Biogenix, 1:200), mouse anti-CD20 (Ventana Medical Systems, neat), mouse anti-CD138 (Ventana Medical Systems, neat), and mouse anti-MUM1 (Dako North America, 1:200). The ultraView Universal DAB detection kit (Ventana Medical Systems) was used with the following primary antibodies: mouse anti-PRDM1/Blimp1 (CNIO, neat) and mouse anti-XBP1s (CNIO, 1:100). Hematoxylin II (Ventana Medical Systems) was applied as the counterstain.
Scoring.
IHC scoring was performed on a scale of 0 to 3+ for intensity and 0 to 4 for percent positive cells (0 indicates no stain; 0.5, < 5%; 1, 5%-25%; 2, 25%-50%; 3, 50%-75%; and 4, 75%-100%). Values were averaged per 3 punches for each case. If tissue microarray (TMA) was inadequate and whole sections were available, scoring was performed using a whole section. Similar in concept to the Allred score that is used for estrogen receptor/progesterone receptor evaluation in breast cancer,38 protein expression scores using chromogenic IHC were calculated by adding average intensity value plus average value for percent positive cells. Thus, calculated scores had a possible range of expression of 0 to 7. Qualitative notation of surface versus cytoplasmic/punctate staining of the HLA-DR stain was made. Images were captured using an Olympus BX45 clinical microscope at 40× (Plan N; Olympus) and a Nikon DS-Fi1 digital CCD camera and DS-L2 imaging controller (Nikon).
Statistics
Relevant statistics were performed using Excel 2007 (Microsoft) and SigmaPlot Version 11.0 (Systat Software). Pearson Product Moment Correlation was used to determine the correlation between expression of 2 genes using Affymetrix data. ANOVA was used to compare MHC II gene expression using Affymetrix data between ABC, GCB, unclassifiable, and PMBCL subtypes of DLBCL. ANOVA was used to compare mean protein expression scores using quantum dots for each marker of interest between MHC II(+), MHC II-punctate, and MHC II(−) DLBCL and PBL cases. The Student t test was used to compare protein expression scores using IHC at different MHC II cutpoints for each marker.
Results
GEP identifies inverse association of plasma cell markers and MHC II
To test whether loss of MHC II is indicative of a plasma cell-like differentiation state in DLBCL, we used gene expression data available on 203 DLBCL cases, which have been profiled using the Affymetrix Human Genome U133A and U133B arrays. A previous study using GEP data demonstrated that expression of B-cell markers CD19, CD20, and CD22 did not vary with MHC II expression.21 Here, we compared MHC II expression with well-known plasma cell markers MUM1, PRDM1/Blimp1, and XBP1s. As described in “GEP data analysis,” an average MHC II gene expression was calculated for each case and compared with MUM1, PRDM1/Blimp1, and XBP1s across all 203 cases. A Pearson product moment correlation was used to demonstrate a significant inverse correlation between MHC II and MUM1 gene expression (r = −0.285, P = .000037), PRDM1/Blimp1 gene expression (r = −0.180, P = .0103), and XBP1s gene expression (r = −0.164, P = .0197). In addition, we confirmed positive correlations of the plasma cell markers with one another: MUM1 and PRDM1/Blimp1 (r = 0.288, P = .0000311), MUM1 and XBP1s (r = 0.366, P = .0000000777), and PRDM1/Blimp1 and XBP1s (r = 0.281, P = .0000480).
MHC II gene expression is significantly lower in ABC-DLBCL than the GCB-DLBCL subtype
Having shown that MHC II expression was inversely correlated with MUM1, and given that MUM1 expression is associated with the ABC subtype of DLBCL,6,39,40 we next compared gene expression of MHC II across DLBCL subtypes using the Affymetrix gene expression data. The 203 DLBCL cases were classified into subtypes as ABC-DLBCL (n = 75), GCB-DLBCL (n = 82), unclassifiable-DLBCL (n = 31), and PMBCL (n = 15) using GEP as previously described.7,26 First we confirmed the association of MUM1 with the ABC subtype of DLBCL in our dataset. ANOVA was used to test for differences between all 4 subsets, and MUM1 expression was found to be significantly higher in ABC-DLBCL than GCB-DLBCL, unclassifiable-DLBCL, or PMBCL, as well as significantly higher in unclassifiable-DLBCL than GCB-DLBCL (P < .05). Each of the DLBCL subtypes demonstrated overlapping ranges of MCH II average gene expression (Figure 1). An ANOVA comparison of MHC II average expression between DLBCL subtypes demonstrated that a significant difference was found between ABC-DLBCL and GCB-DLBCL subtypes (P = .015), with higher expression of MHC II in the clinically favorable GCB-DLBCL subtype (Figure 1). We further found that PRDM1/Blimp1 gene expression was significantly lower in GCB-DLBCL than ABC-DLBCL or unclassifiable-DLBCL (P < .05). XBP1s gene expression was significantly lower in GCB-DLBCL than ABC-DLBCL, unclassifiable-DLBCL, or PMBCL (P < .05).
MHC II gene expression varies in ABC, GCB, unclassifiable, and PMBCL subtypes of DLBCL. (A) Expression of MHC II per case, per subtype. Line indicates median. (B) Mean expression of MHC II per subtype. *P < .05.
MHC II gene expression varies in ABC, GCB, unclassifiable, and PMBCL subtypes of DLBCL. (A) Expression of MHC II per case, per subtype. Line indicates median. (B) Mean expression of MHC II per subtype. *P < .05.
DLBCL and PBL display different profiles of CD20, HLA-DR, and PRDM1/Blimp1 expression
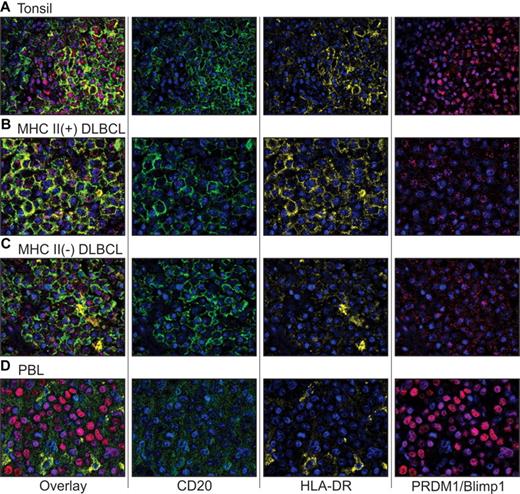
Having shown that MHC II expression is inversely related to the expression of plasma cell markers at the mRNA level, we sought to determine whether this translated into a relationship between MHC II and a transient plasma cell-like differentiation state at the protein level. We used multiplexed quantum dot fluorescent IHC to investigate the colocalization of MHC II (HLA-DR) with a B-cell marker (CD20) or a plasma cell marker (PRDM1/Blimp1). Evaluation of reactive tonsil demonstrated various intensities for CD20, HLA-DR, and PRDM1/Blimp1, in various combinations in different B-cell subsets (Figure 2A). Strong CD20 and HLA-DR were generally concurrent on the surface of B cells in the germinal center and mantle zone. Nuclear PRDM1/Blimp1 was seen in CD20(−), HLA-DR(−) plasma cells within the germinal center, as well as intrafollicular regions. Occasional germinal center cells demonstrated concurrent CD20 and PRDM1/Blimp1, either with or without HLA-DR expression, and presumably represent rare cells on the cusp of switching from mature B cells to plasma cells. In contrast to the various combinations seen in the different cell populations of tonsil tissue, the expression patterns of CD20, HLA-DR, and PRDM1/Blimp1 were more homogeneous within lymphoma cases.
Quantum dot IHC for expression of CD20, MHC II (HLA-DR), and PRDM1/Blimp1 in tonsil, DLBCL, and PBL. (A) Tonsil control. (B) MHC II(+) DLBCL. (C) MHC II(−) DLBCL. (D) PBL. Multiplexed quantum dot IHC was performed as described in “Quantum dot fluorescent IHC.” Images (original magnification ×40) demonstrate the composite wavelength image (overlay), followed by 2-color overlays of the unmixed spectral components (CD20, HLA-DR, and PRDM1/Blimp1) depicting individual quantum dot analytes and nuclear counterstain (DAPI). It should be noted that the imaging software artificially increases intensity to match the dynamic range of the display, so that some proteins scored as negative by eye demonstrate an artifactual dusting in the overlay images. For detailed methods on image acquisition, please see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Quantum dot IHC for expression of CD20, MHC II (HLA-DR), and PRDM1/Blimp1 in tonsil, DLBCL, and PBL. (A) Tonsil control. (B) MHC II(+) DLBCL. (C) MHC II(−) DLBCL. (D) PBL. Multiplexed quantum dot IHC was performed as described in “Quantum dot fluorescent IHC.” Images (original magnification ×40) demonstrate the composite wavelength image (overlay), followed by 2-color overlays of the unmixed spectral components (CD20, HLA-DR, and PRDM1/Blimp1) depicting individual quantum dot analytes and nuclear counterstain (DAPI). It should be noted that the imaging software artificially increases intensity to match the dynamic range of the display, so that some proteins scored as negative by eye demonstrate an artifactual dusting in the overlay images. For detailed methods on image acquisition, please see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
DLBCL cases were classified as MHC II(+) (n = 8), MHC II-punctate (intracellular, mislocalized nonsurface expression; n = 13), or MHC II(−) (n = 7). DLBCL tumor cells were uniformly positive for surface expression of CD20, with variable expression of HLA-DR, and limited nuclear PRDM1/Blimp1 expression (Table 1; Figures 2B-C and 3). In cases designated as MHC II-punctate, HLA-DR staining was seen in an intracellular cytoplasmic punctate pattern alone or occasionally with combined cytoplasmic and surface staining. PRDM1/Blimp1 expression was generally limited in DLBCL cases and demonstrated a weak and somewhat scattered pattern in the nucleus.
Summary of quantum dot IHC
| . | CD20 . | HLA-DR . | Blimp1 . |
|---|---|---|---|
| MHC II(+) DLBCL (n = 8) | |||
| Range | 110.0-185.0 | 95.0-160.0 | 0.0-45.0 |
| Median | 150.0 | 127.5 | 30.0 |
| Mean | 150.6 | 124.4 | 25.0 |
| MHC II-punctate DLBCL (n = 13) | |||
| Range | 50.0-275.0 | 0.0-140.0 | 0.0-70.0 |
| Median | 150.0 | 10.0 | 0.0 |
| Mean | 162.3 | 37.7 | 12.3 |
| MHC II(−) DLBCL (n = 7) | |||
| Range | 110.0-280.0 | 0.0-0.0 | 0.0-75.0 |
| Median | 180.0 | 0.0 | 0.0 |
| Mean | 179.3 | 0.0 | 18.6 |
| PBL (n = 13) | |||
| Range | 0.0-30.0 | 0.0-0.0 | 0.0-110.0 |
| Median | 0.0 | 0.0 | 75.0 |
| Mean | 2.0 | 0.0 | 25.0 |
| . | CD20 . | HLA-DR . | Blimp1 . |
|---|---|---|---|
| MHC II(+) DLBCL (n = 8) | |||
| Range | 110.0-185.0 | 95.0-160.0 | 0.0-45.0 |
| Median | 150.0 | 127.5 | 30.0 |
| Mean | 150.6 | 124.4 | 25.0 |
| MHC II-punctate DLBCL (n = 13) | |||
| Range | 50.0-275.0 | 0.0-140.0 | 0.0-70.0 |
| Median | 150.0 | 10.0 | 0.0 |
| Mean | 162.3 | 37.7 | 12.3 |
| MHC II(−) DLBCL (n = 7) | |||
| Range | 110.0-280.0 | 0.0-0.0 | 0.0-75.0 |
| Median | 180.0 | 0.0 | 0.0 |
| Mean | 179.3 | 0.0 | 18.6 |
| PBL (n = 13) | |||
| Range | 0.0-30.0 | 0.0-0.0 | 0.0-110.0 |
| Median | 0.0 | 0.0 | 75.0 |
| Mean | 2.0 | 0.0 | 25.0 |
Data are range and averages for CD20 (B-cell marker), HLA-DR (MHC II), and Blimp1 (plasma cell marker) in 28 cases of DLBCL divided by MHC II status, and 15 cases of PBL.
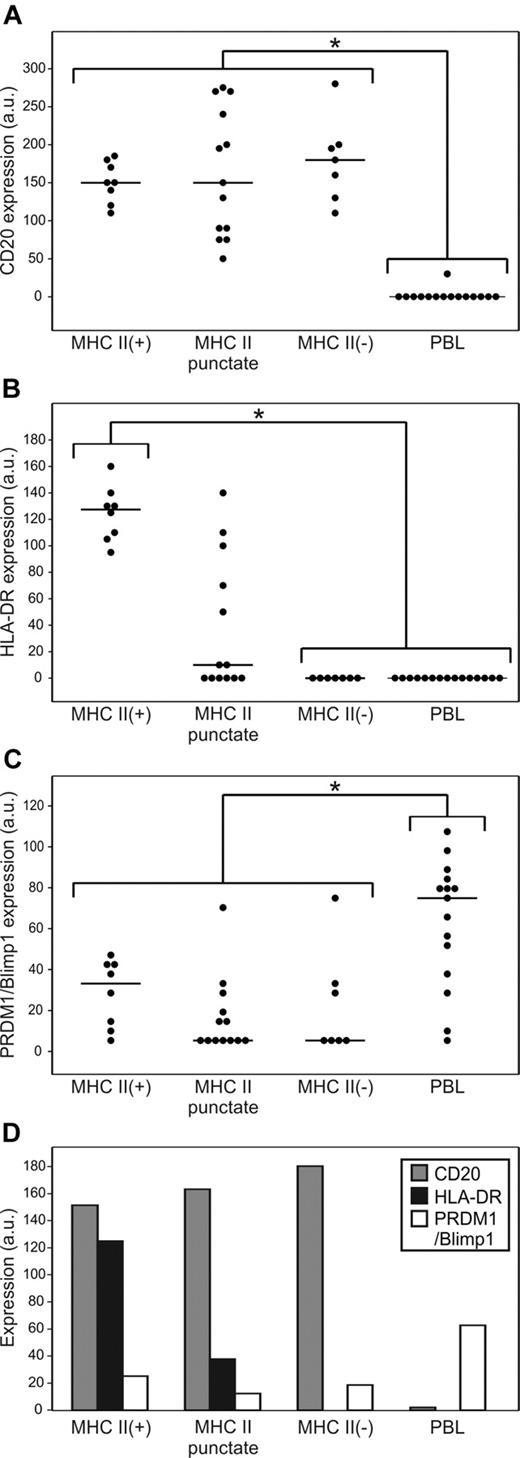
DLBCL and PBL display markedly different profiles of CD20, HLA-DR, and PRDM1/Blimp1 expression. Individual protein expression scores (a.u. indicates arbitrary units) using quantum dot IHC were calculated per marker, per case, as described in “Quantum dot fluorescent IHC” (possible range, 0-300). Line indicates median value. *P < .05. (A) CD20 expression is significantly lower in PBL compared to all 3 categories of DLBCL. (B) HLA-DR expression is significantly lower in PBL and MHCII (−) DLBCL compared to MHCII (+) or MHCII-puncture DLBCL. (C) PRDM1/Blimpl is significantly higher in PBL compared to all 3 categories of DLBCL. (D) Mean scores of CD20, HLA-DR, and PRDM1/Blimp1 in MHC II(+), MHC II-punctate, and MHC II(−) DLBCL and PBL.
DLBCL and PBL display markedly different profiles of CD20, HLA-DR, and PRDM1/Blimp1 expression. Individual protein expression scores (a.u. indicates arbitrary units) using quantum dot IHC were calculated per marker, per case, as described in “Quantum dot fluorescent IHC” (possible range, 0-300). Line indicates median value. *P < .05. (A) CD20 expression is significantly lower in PBL compared to all 3 categories of DLBCL. (B) HLA-DR expression is significantly lower in PBL and MHCII (−) DLBCL compared to MHCII (+) or MHCII-puncture DLBCL. (C) PRDM1/Blimpl is significantly higher in PBL compared to all 3 categories of DLBCL. (D) Mean scores of CD20, HLA-DR, and PRDM1/Blimp1 in MHC II(+), MHC II-punctate, and MHC II(−) DLBCL and PBL.
PBL cases were uniformly negative for CD20 and HLA-DR, and generally positive for PRDM1/Blimp1 expression (Table 1; Figures 2D and 3). Only one case of 15 demonstrated weak CD20 staining in approximately 30% of cells. This is in agreement with previous studies.8,41 Expression of HLA-DR was completely absent from all 15 cases of PBL. Occasional nontumor small B cells demonstrated staining for CD20 and HLA-DR but were excluded from scoring of the tumor. PRDM1/Blimp1 expression in PBL demonstrated a homogeneous nuclear staining pattern coincident with DAPI, unlike the weak quality of scattered, dot-like PRDM1/Blimp1 staining seen in DLBCL.
ANOVA was used to test for significant differences in median protein expression scores for each marker between the 4 groups: MHC II(+), MHC II-punctate, and MHC II(−) DLBCL, and PBL (Figure 3A-C). CD20 quantum dot expression scores were significantly different for PBL relative to MHC II(+), MHC II-punctate, and MHC II(−) DLBCL (P < .05). HLA-DR quantum dot expression scores were not significantly different for MHC II(−) DLBCL and PBL, and were significantly different for MHC II(+) DLBCL relative to MHC II(−) DLBCL and PBL (P < .05). HLA-DR scores for MHC II-punctate cases were intermediate between MHC II(+) and MHC II(−) cases, and not statistically different from either group. Quantum dot expression scores for PRDM1/Blimp1 were significantly different for PBL relative to MHC II(+), MHC II-punctate, and MHC II(−) DLBCL (P < .01). PRDM1/Blimp1 expression did not vary significantly between cases of MHC II(+), MHC II-punctate, and MHC II(−) DLBCL. Our results that PRDM1/Blimp1 is limited in DLBCL and more prevalent in PBL confirm previous studies.42,43 As summarized in Figure 3D, MHC II(+) DLBCL cases are characterized by strong expression of CD20 and HLA-DR, with limited expression of PRDM1/Blimp1. MHC II-punctate and MHC II(−) DLBCL cases have a similar CD20/PRDM1/Blimp1 profile but demonstrate reduced and negative expression of HLA-DR, respectively. PBL cases are characterized by loss of CD20 and HLA-DR expression, with strong expression of PRDM1/Blimp1. Thus, the profile of MHC II(−) DLBCL cases is intermediate between that of MHC II(+) DLBCL and PBL.
B-cell markers and morphology do not correlate with MHC II
To further study whether MHC II(−) DLBCL cases are farther along the differentiation spectrum than MHC II(+) cases, we used a tissue microarray (TMA) of a subset of DLBCL cases that have been classified into different levels of MHC II expression by GEP.6,21 The hematoxylin and eosin–stained section of the TMA was examined for tumor morphology. Of the 40 cases on the TMA, 36 were centroblastic and 4 were immunoblastic. Of these, all 4 cases fell into the upper 50% of MHC II expression and were thus not associated with MHC II loss. Correlations with morphology may be difficult to appreciate as MHC II(−) cases were under-represented on the TMA.
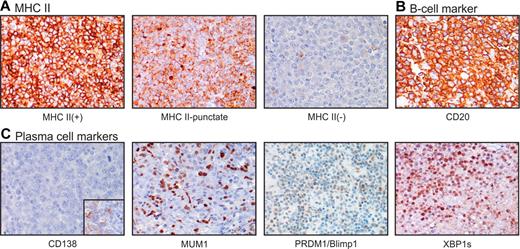
The TMA was successfully stained for B-cell markers HLA-DR (MHC II; 39 of 40 cases) and CD20 (37 of 40). Figure 4 demonstrates that each IHC stain was successful but that the full range of expression (intensity and percentage of positive cells) varied by marker (see also Table 2). HLA-DR IHC ranged from 0.0 to 7.0 and generally correlated with MHC II status by GEP (Table 1), similar to cases previously described.21 Generally, MHC II(+) cases demonstrated typical staining of HLA-DR at the cytoplasmic membrane. However, a unique pattern of HLA-DR expression was also observed, which was characterized by a mislocalized intracellular/punctate expression, rather than the usual surface expression (Figure 4A). This pattern was observed in 15 of the 40 cases, with 2 cases being in the 10% to 25% range of MHC II expression, 3 cases in the 25% to 50% range, and 10 cases in the more than 50% range.
Expression of MHC II, B-cell markers, and plasma cell markers in DLBCL. Chromogenic IHC was performed as described in “Chromogenic IHC” to visualize proteins as marked. Original magnification ×40. The full range of expression (intensity and percentage of positive cells) varied for each protein; images selected show the upper range of expression for that marker. (A) MHCII marker HLA-DR. (B) B-cell marker CD20, and (C) plasma-cell markers CD138, MUMI, PRDM1/Blimp1, XBP1s. Inset: positive staining for CD138 in tonsil control.
Expression of MHC II, B-cell markers, and plasma cell markers in DLBCL. Chromogenic IHC was performed as described in “Chromogenic IHC” to visualize proteins as marked. Original magnification ×40. The full range of expression (intensity and percentage of positive cells) varied for each protein; images selected show the upper range of expression for that marker. (A) MHCII marker HLA-DR. (B) B-cell marker CD20, and (C) plasma-cell markers CD138, MUMI, PRDM1/Blimp1, XBP1s. Inset: positive staining for CD138 in tonsil control.
Summary of chromogenic IHC
| MHCII status . | HLA-DR . | CD20 . | CD138 . | MUM1 . | Blimp1 . | XBP1s . |
|---|---|---|---|---|---|---|
| Overall (n = 40) | ||||||
| Range | 0.0-7.0 | 2.3-7.0 | 0.0-0.5 | 0.0-5.0 | 0.0-3.8 | 0.0-5.5 |
| Median | 5.0 | 6.0 | 0.0 | 2.3 | 0.7 | 2.2 |
| Mean | 4.4 | 5.8 | 0.0 | 2.4 | 0.9 | 2.0 |
| Low 10% (n = 2) | ||||||
| Range | NA | 6.0-6.3 | NA | 1.5-5.0 | 0.0-1.0 | NA |
| Median | 0.0 | 6.2 | 0.0 | 3.3 | 0.5 | 0.0 |
| Mean | 0.0 | 6.2 | 0.0 | 3.3 | 0.5 | 0.0 |
| 10%-25% (n = 6) | ||||||
| Range | 0.0-6.0 | 3.5-7.0 | NA | 2.0-5.0 | 0.0-2.5 | 0.8-5.5 |
| Median | 5.0 | 7.0 | 0.0 | 4.7 | 0.7 | 1.5 |
| Mean | 3.4 | 5.7 | 0.0 | 4.3 | 1.1 | 2.4 |
| 25%-50% (n = 7) | ||||||
| Range | 0.0-7.0 | 3.0-7.0 | NA | 0.0-5.0 | 0.7-3.8 | 0.0-4.7 |
| Median | 5.0 | 5.7 | 0.0 | 2.5 | 2.3 | 2.5 |
| Mean | 3.6 | 5.6 | 0.0 | 2.1 | 2.2 | 2.3 |
| Upper 50% (n = 25) | ||||||
| Range | 0.0-7.0 | 2.3-7.0 | 0.0-0.5 | 0.0-4.3 | 0.0-1.6 | 0.0-5.0 |
| Median | 5.7 | 6.0 | 0.0 | 2.2 | 0.4 | 2.2 |
| Mean | 5.2 | 5.9 | 0.0 | 2.0 | 0.6 | 2.0 |
| MHCII status . | HLA-DR . | CD20 . | CD138 . | MUM1 . | Blimp1 . | XBP1s . |
|---|---|---|---|---|---|---|
| Overall (n = 40) | ||||||
| Range | 0.0-7.0 | 2.3-7.0 | 0.0-0.5 | 0.0-5.0 | 0.0-3.8 | 0.0-5.5 |
| Median | 5.0 | 6.0 | 0.0 | 2.3 | 0.7 | 2.2 |
| Mean | 4.4 | 5.8 | 0.0 | 2.4 | 0.9 | 2.0 |
| Low 10% (n = 2) | ||||||
| Range | NA | 6.0-6.3 | NA | 1.5-5.0 | 0.0-1.0 | NA |
| Median | 0.0 | 6.2 | 0.0 | 3.3 | 0.5 | 0.0 |
| Mean | 0.0 | 6.2 | 0.0 | 3.3 | 0.5 | 0.0 |
| 10%-25% (n = 6) | ||||||
| Range | 0.0-6.0 | 3.5-7.0 | NA | 2.0-5.0 | 0.0-2.5 | 0.8-5.5 |
| Median | 5.0 | 7.0 | 0.0 | 4.7 | 0.7 | 1.5 |
| Mean | 3.4 | 5.7 | 0.0 | 4.3 | 1.1 | 2.4 |
| 25%-50% (n = 7) | ||||||
| Range | 0.0-7.0 | 3.0-7.0 | NA | 0.0-5.0 | 0.7-3.8 | 0.0-4.7 |
| Median | 5.0 | 5.7 | 0.0 | 2.5 | 2.3 | 2.5 |
| Mean | 3.6 | 5.6 | 0.0 | 2.1 | 2.2 | 2.3 |
| Upper 50% (n = 25) | ||||||
| Range | 0.0-7.0 | 2.3-7.0 | 0.0-0.5 | 0.0-4.3 | 0.0-1.6 | 0.0-5.0 |
| Median | 5.7 | 6.0 | 0.0 | 2.2 | 0.4 | 2.2 |
| Mean | 5.2 | 5.9 | 0.0 | 2.0 | 0.6 | 2.0 |
Data are overall range and averages for B-cell (CD20, HLA-DR) and plasma cell (CD138, MUM1, Blimp1, XBP1) markers in 40 cases of DLBCL as well as range and averages for DLBCL cases divided by MHC II status based on GEP.
NA indicates not applicable.
In support of previous GEP analysis,21 protein expression of B-cell marker CD20 did not correlate with low or absent gene expression of MHC II in DLBCL (Table 1). Cases were uniformly positive for CD20 staining at the cytoplasmic membrane, with no cases having less than a 1 for intensity, or less than 25% positive cells. Protein expression of B-cell markers Oct2, Bob1, and Pax5 varied across all cases, regardless of MHC II status (D.R.F., S.T.W., and L.M.R., unpublished observation, December 10, 2011). In addition, a review of a previous study that scored the same set of cases for expression of B-cell marker Bcl-6 did not suggest any correlation with MHC II expression.44
Plasma cell markers are enriched in MHC II(−) DLBCL cases
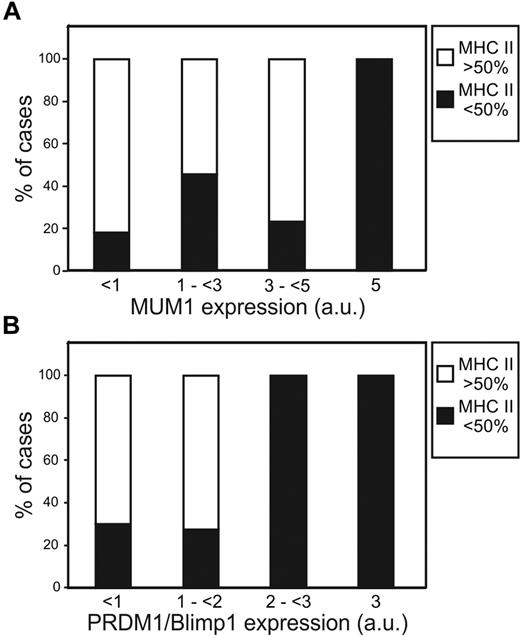
The TMA was successfully stained for plasma cell markers CD138 (40 of 40 cases), MUM1 (39 of 40), PRDM1/Blimp1 (35 of 40), and XBP1s (33 of 40; Figure 4; Table 2). MUM1, PRDM1/Blimp1, and XBP1s demonstrated nuclear staining. The DLBCL cases were uniformly negative for CD138 protein expression, with only one case demonstrating limited intensity in a fraction of cells. However, IHC for MUM1, PRDM1/Blimp1, and XBP1s did show some inverse association with MHC II levels classified by GEP (Table 1). When MUM1 protein expression scores by IHC were compared with MHC II levels as classified by GEP, they demonstrated an inverse trend. Lower MUM1 scores were predominated by cases with MHC II more than 50% by GEP, with higher MUM1 scores predominated by MHC II less than 50% (Figure 5A). A t test comparing MUM1 IHC expression scores at different cutoffs of MHC II by GEP gave P = .08 for the 50% cutoff and P = .01 for the 25% cutoff. The expression of plasma cell marker PRDM1/Blimp1 was somewhat limited. Lower PRDM1/Blimp1 scores were predominated by cases with MHC II more than 50% by GEP, with higher PRDM1/Blimp1 scores predominated by MHC II less than 50% (Figure 5B). When PRDM1/Blimp1 IHC scores were compared between cases with more than 50% MHC II or less than 50% MHC II cutoffs by GEP, there was a statistically significant inverse association (P = .04). Scores were generally low for plasma cell marker, XBP1s, and did not appear to vary with MHC II expression. However, one case did stand out as having strong staining of XBP1s in a majority of cells (score = 5.5), and this case was classified at the MHC II 10% to 25% level by GEP. The same case was further marked by limited expression of B-cell marker CD20 and negative expression of B-cell marker Pax5 (D.R.F., S.T.W., and L.M.R., unpublished observation, December 10, 2011). This is similar to the phenotype described in PBL by Montes-Moreno et al.42 We reviewed this case to determine whether it demonstrated an immunoblastic/plasmablastic morphology, which might suggest it should be reclassified as PBL, but centroblastic morphology typical of DLBCL was confirmed.
Expression of MUM1 and PRDM1/Blimp1 demonstrates an inverse association with MHC II. Protein expression scores (a.u. indicates arbitrary units) using chromogenic IHC were calculated as described in “Chromogenic IHC” (possible range, 0-7) and compared between cases of DLBCL with MHC II < 50% or > 50% as defined by GEP. (A) Cases with MUMI protein expression of 5 a.u., were all in the bottom 50% of MHCII expression. (B) Cases with PRDM1/Blimp1 protein expression of 2-3 were all in the bottom 50% of MHCII expression.
Expression of MUM1 and PRDM1/Blimp1 demonstrates an inverse association with MHC II. Protein expression scores (a.u. indicates arbitrary units) using chromogenic IHC were calculated as described in “Chromogenic IHC” (possible range, 0-7) and compared between cases of DLBCL with MHC II < 50% or > 50% as defined by GEP. (A) Cases with MUMI protein expression of 5 a.u., were all in the bottom 50% of MHCII expression. (B) Cases with PRDM1/Blimp1 protein expression of 2-3 were all in the bottom 50% of MHCII expression.
Discussion
In this study, we asked whether DLBCL cases that have lost MHC II expression, a phenotype associated with poor prognosis, are farther along the B-cell differentiation spectrum than cases that have continued MHC II expression. We used a well-characterized case series of DLBCL with no consistent genetic or epigenetic mechanism of decreased MHC II expression. We show that MHC II(−) DLBCLs more often have a late-B cell immunophenotype, including expression of MUM1, PRDM1/Blimp1, and XBP1s, and are more commonly of the ABC subtype, however more differentiated than ABC-DLBCL as a group by sharing a lack of HLA-DR with PBL (whereas ABC-DLBCL may be positive or negative). These MHC II(−) DLBCL cases, however, still express CD20 and lack CD138 as is typical of mature B cells, as opposed to fully differentiated plasma cells, which are CD20(−) and CD138(+). In further support of this late B-cell concept, we previously measured the expression of genes that are B-cell lineage-related or prognostically important in DLBCL in cases of MHC II(+) and MHC II(−) DLBCL and PBL. We showed that fewer genes differ between MHC II(−) DLBCL and PBL than between MHC II(+) DLBCL and PBL.45 In addition, a previous study by our group looked at histologic subtypes for different quartile splits of MHC II (HLA-DRA).21 In that study, although cases with decreased HLA-DRA fell into all histologic subtypes, plasmablastic histology did make up a higher percentage of cases with limited MHC II than cases with abundant MHC II. In the current study, no obvious correlations with plasmablastic or immunoblastic morphology were found; however, only 2 cases on the TMA were in the lowest 10% and 6 in the lowest 25% of expression by GEP. Taken together, these findings suggest that, although MHC II(−) DLBCL cases do not present a fully differentiated plasma cell phenotype, MHC II loss may arise from cells farther along the differentiation continuum than MHC II(+) cases. We further propose that MHC II(−) cases may represent the interface between DLBCL and plasmablastic lymphoma. Previously, alteration of BLIMP1 expression was associated with lymphomagenesis by potentially disrupting B-cell differentiation.46 Other authors have asserted a model in which DLBCL overlaps with PBL, with the latter being farther along the terminal B-cell differentiation pathway.47 In addition, short-lived or transitory cells intermediate in the B-cell/plasma cell spectrum have been described, which express partial signatures of both B cells and plasma cells.48,49 Because MHC II expression is only completely extinguished in fully mature plasma cells, it is possible that MHC II(−) DLBCLs arise from an infrequent, transitory subset of pre-plasmablasts or intermediate plasma cells, which may overlap with the ABC-DLBCL subtype.
Loss of MHC class II and class I in B-cell malignancies correlates with poor outcome in nearly all studies to date. Of particular interest is the lower expression of MHC II in the clinically unfavorable ABC-DLBCL compared with GCB-DLBCL subtype using Affymetrix GEP data. A previous study by our group compared MHC II gene expression between DLBCL subtypes using Lymphochip data on many of the same cases of DLBCL.26 In that former study including DLBCL and PMBCL, we also demonstrated that the level of MHC II gene expression was higher in GCB-DLBCL than in ABC-DLBCL, unclassifiable-DLBCL, and PMBCL (these 3 latter groups were similar in expression to each other). In the current study, the number of unclassifiable-DLBCL and PMBCL cases available with Affymetrix data did not allow for sufficient power to detect significant differences in these groups; however, a similar trend was noted. To our knowledge, the present study is the first description of PBL being uniformly negative for MHC II. This finding, coupled with the expression of PRDM1/Blimp1, suggests transcriptional, rather than genetic, regulation as the mechanism of MHC II loss in PBL. Given the association of lost MHC II with poor prognosis in DLBCL, we suggest that the consequent effects on tumor immunosurveillance may also contribute to the dismal outcome of PBL patients. Loss of MHC II is also a frequent event in another clinically unfavorable subset of DLBCL, involving immune-privileged sites, such as testes or brain.13 Such immune-privileged cases have also been shown to demonstrate ABC-DLBCL characteristics, such as increased levels of MUM1 expression.50
In addition, we observed the phenomenon of mislocalized punctate MHC II staining in B-cell lymphomas. Such intracellular MHC II staining is not observed in any B-cell population in benign tissues, including lymph node, spleen, or bone marrow (L.M.R. and T.M.G., unpublished observation, 2011). We had previously published our observation that 2 cases with low mRNA levels of HLA-DR had a punctate cytoplasmic staining pattern of HLA-DR protein in lymphoma cells.21 These 2 cases with this unique staining also had very few tumor-infiltrating T cells.21 In the present study, we show that punctate MHC II expression is also seen in cases with abundant MHC II mRNA. In addition, many cases of PMBCL from a previous study26 had this punctate cytoplasmic staining pattern (L.M.R. and T.M.G., unpublished observation, 2011). Because MHC II proteins traffic through the endosomal pathway for peptide loading before final presentation on the cell surface of B cells, it is possible that the cytoplasmic localization is indicative of an alteration of the trafficking process and that HLA-DR is not fully available for antigen presentation on the cell surface in these cases. This could provide a reason why some patients with normal levels of MHC II mRNA still have a poor clinical outcome. Studies by 2 other groups have mentioned nonsurface HLA-DR protein expression as an aside in DLBCL, referring to “equivocal” staining with “focal cytoplasmic positivity”51 and “predominantly membranous, but occasionally cytoplasmic” positive staining.22 A study by Diepstra et al provided additional detail, showing that lack of surface membrane MHC II is common in classical Hodgkin lymphoma (cHL).28 In that study using IHC, Hodgkin cases that demonstrated complete loss of MHC II were grouped with cases that expressed MHC II but lacked the normal membranous staining pattern, and together these 41.4% of cases were considered MHC II(−). MHC II loss was again associated with poor survival.28 These combined studies indicate that the mislocalized punctate staining pattern is probably associated with poor outcome in a variety of B-cell malignancies.
The results reported here imply a spectrum of MHC II expression on DLBCL that may be related to a later stage of differentiation (with complete loss in PBL; Figure 6). Whether these cases represent a normal, brief phase of differentiation in which MHC II is down-regulated in B cells before full plasma cell differentiation or is an abnormal phenomenon arising during tumorigenesis cannot yet be determined. However, these observations provide a possible unifying concept that may contribute to the poor outcome reported in all MHC II(−) B-cell tumors. Finally, we propose that antigen presentation is a critical pathway deserving of targeted therapeutic development.
Proposed model of relationship between MHC II expression and DLBCL differentiation. Within the spectrum of DLBCL, there is variation in the expression of markers of plasma cell differentiation. We propose that MHC II expression inversely correlates with markers of plasma cell differentiation and is more commonly lost in cases further along the spectrum toward the plasmablastic lymphomas.
Proposed model of relationship between MHC II expression and DLBCL differentiation. Within the spectrum of DLBCL, there is variation in the expression of markers of plasma cell differentiation. We propose that MHC II expression inversely correlates with markers of plasma cell differentiation and is more commonly lost in cases further along the spectrum toward the plasmablastic lymphomas.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Catherine Rangel, Yvette Frutiger, and Robin Roberts for technical assistance and expertise and the Lymphoma/Leukemia Molecular Profiling Research Group for help in producing the large and well-annotated datasets that are providing new insights into the molecular pathology of NHL.
This work was supported by the American Cancer Society (RSG0605501L1B) and in part by Ventena Medical Systems Inc (research funding; K.A.V., K.E.G., and T.M.G.). S.T.W. was supported in part by the National Cancer Institute (T32 training grant NCI-T32 CA09213).
National Institutes of Health
Authorship
Contribution: S.T.W. analyzed and interpreted data, made the figures, and wrote the manuscript; L.M.R., T.M.G., and J.T.-F. contributed case materials; L.M.R., D.R.F., and P.B. performed scoring of cases; S.T.W., K.A.V., K.E.G., and B.J.G.-G. performed laboratory work; L.M.R. was the principal investigator, designed research, interpreted data, and wrote the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: K.A.V., P.B., K.E.G., and T.M.G. are employees of Ventana Medical Systems, which provided reagents and performed Q-dot staining free of charge for this project. The remaining authors declare no competing financial interests.
Correspondence: Lisa M. Rimsza, Department of Pathology, 1501 N Campbell Ave, Box 245043, Tucson, AZ 85724-5043; e-mail: lrimsza@email.arizona.edu.