Abstract
The efficiency of humoral immune responses depends on the selective outgrowth of B cells and plasmacells that produce high affinity antibodies. The factors responsible for affinity maturation of B cell clones in the germinal center (GC) have been well established but selection mechanisms that allow clones to enter the GC are largely unknown. Here we identify apoptosis, regulated by the proapoptotic BH3-only member Noxa (Pmaip1), as a critical factor for the selection of high-affinity clones during B cell expansion after antigen triggering. Noxa is induced in activated B cells, and its ablation provides a survival advantage both in vitro and in vivo. After immunization or influenza infection, Noxa−/− mice display enlarged GCs, in which B cells with reduced antigen affinity accumulate. As a consequence, Noxa−/− mice mount low affinity antibody responses compared with wild-type animals. Importantly, the low affinity responses correlate with increased immunoglobulin diversity, and cannot be corrected by booster immunization. Thus, normal elimination of low affinity cells favors outgrowth of the remaining high-affinity clones, and this is mandatory for the generation of proper antibody responses. Manipulation of this process may alter the breadth of antibody responses after immunization.
Introduction
The humoral immune system provides long-lasting immunity against invading pathogens and their products via the generation of neutralizing antibodies and by the formation of memory B cells. The naive B-cell pool is able to recognize a vast number of different molecular patterns via a huge set of clonally unique B-cell receptors (BCRs). Specificity in the response to a given pathogen derives from clonal expansion of only those cells that bind antigen with a certain minimal affinity.1 Specificity is improved during T cell–dependent responses, in which a limited number of B cell clones is allowed to seed the germinal center (GC) where they increase affinity by mutating the variable regions of their immunoglobulin genes. Eventually, B cells with the highest affinity for antigen are selected and subsequently differentiate into memory cells or antibody-secreting plasma cells. Molecular processes regulating selection of B cells in the GC have been studied extensively,2-5 but the mechanisms which control the access of B cells to the GC are largely unclear.
The BCRs can mediate differential signaling in vivo,6,7 and provide the primary B-cell activating signal. However, BCR signal strength alone is not sufficient to determine the outcome of the response. Studies using B cells from BCR transgenic mice showed that under noncompetitive conditions BCR affinity had little effect on the number of cells contributing to the overall response, nor on the amount of antibody produced. However, after both T cell–dependent and independent immunizations, high-affinity B cells already predominated at the earliest stages of the responses when high and low affinity BCR transgenic B cells competed for the same antigen within one mouse.8-10 Interestingly, hypermutation rates were not altered between high and low-affinity cells,8 suggesting that additional selection mechanisms besides affinity maturation in the GC play a role in B-cell selection. Control of apoptosis may be a primary regulatory mechanism because after activation in vivo, high affinity B cells have a survival advantage over low affinity cells, without an apparent difference in division rates.11
Both death-receptor mediated apoptosis and the mitochondrial death pathway have been implicated in the regulation of expansion of antigen-stimulated B and T lymphocytes.12,13 The intracellular cell death pathway is modulated by the Bcl-2 family of pro and antiapoptotic proteins, which control mitochondrial outer membrane integrity14 and determine whether apoptosis is triggered. For this reason their expression is carefully regulated, both at the transcriptional and posttranslational level. Previously, we have identified mitochondrial apoptosis, regulated by proapoptotic Noxa (Pmaip1) and its antagonist Mcl-1, as an important regulatory mechanism for selection of dividing T cells under conditions where they have to compete for nutrients and cytokines in vitro.15 More recently we demonstrated that Noxa is an important regulator for in vivo selection of high-affinity T cell clones. Murine T cells up-regulate both Noxa and Mcl-1 on activation. Mcl-1 protein stability relied on T-cell receptor (TCR) signaling strength in an IL-2–dependent manner.16 Vikstrom et al recently showed that Mcl-1 is crucial for the survival B cells early after activation, whereas Bcl-XL was only essential later.17 Importantly, as the effect of Mcl-1 ablation can be already detected very early in the immune response, it was suggested that the dependence on an Mcl-1–regulated survival pathway precedes GC selection. This notion is supported by the observation that hypermutation rates were unaffected in mice lacking 1 Mcl-1 allele.17 Only very few B cells remained after Mcl-1 deletion, complicating analysis of the importance of the Mcl-1/Noxa axis for the survival of antigen-stimulated B cells. However, Noxa−/− mice have normal lymphoid development, providing the opportunity to further define the contribution of the Mcl-1/Noxa pair in B-cell selection.
We report here that elimination of activated, low affinity B cells was perturbed in the absence of Noxa, leading to the persistence of more GC-seeding clones and inferior antibody responses. Importantly, hypermutation rates were not significantly affected in these animals, indicating a selection phase preceding or independent of affinity maturation. We identify Noxa-regulated apoptosis as a novel selection mechanism during B-cell expansion, which limits the amount of low-affinity clones allowed to participate in an immune response, thereby generating space for the outgrowth of high-affinity clones.
Methods
All materials and methods concerning mice, flow cytometry, RT-MLPA analyses, immunoblot, RT-PCR, immunohistochemistry, and statistical analyses were as previously described,16 and are detailed in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). For the current work, the following specific methods were applied. All animal experiments were performed after approval from the Academic Medical Center of Amsterdam's animal ethics committee.
Cell culture and ELISA
Splenic B cells were purified to > 95% purity using the MACS cell separation system (Myltenyi) and anti-CD19 microbeads (Myltenyi). Cells were stimulated in RPMI with 10% fetal calf serum (FCS) with soluble anti-IgM (HB88) plus lipopolysaccharide (LPS; Escherichia coli 0111:B4, Sigma-Aldrich). Cell division was analyzed by CFSE (Molecular Probes). B-cell subsets were analyzed by CD138, CD38, and GL7 staining. Isotype specific ELISA was performed on culture supernatant or serum. Macsisorb ELISA plates (NUNC) were coated O/N with TNP-BSA (Biosearch Technologies), phycoerythrin (PE; Sigma-Aldrich), influenza virus stock, or isotype-specific capture antibodies (Southern Biotech). Antibody levels were determined using biotinylated detection antibodies (Southern Biotech), using isotype specific antibodies (Southern Biotech) as standards. Biotinylated antibodies were detected using streptavidin-labeled alkaline phosphatase (Southern Biotech) and SigmaFAST pNPP tablets (Sigma-Aldrich). Optical density was determined with a photospectrometer. KD was determined using Graphpad Prism 5.0 software (GraphPad).
Immunization, viral infection, and BrdU labeling
Mice were immunized intraperitoneally with either 50 μg TNP-KLH 30:1 (Biosearch Technologies) in Alum (Imject Alum; Thermo Fisher Scientific), IFA (Thermo Fisher Scientific), 100 μg PE (Sigma-Aldrich) in alum, or with 50 μg NP-CGG (15:1) in alum. Influenza A A/PR8/34 strain (H1N1) was generated in LLC-MK2 cells. TCID50 was determined in B6 mice. Mice were infected intranasally with 10× TCID50 under general anesthesia. Ten days after infection, mice were injected with a single dose of BrdU (5 mg). Two hours after injection, spleens were isolated and BrdU incorporation was visualized using anti-BrdU antibodies (BD Biosciences).
Biacore analysis
A sensor chip CM5 was coated with αIgM and αIgG1 (Southern Biotech) to approximately 3800 RU according to procedures recommended by the manufacturer. Flow buffer was PBS; 0.005% P20 at 30 μL/min. Mouse IgG or IgM was captured from sera diluted 1:50 in flow buffer before injection. Binding to TNP-BSA of captured antibodies was tested by duplicate injections of 0, 5, 10, 50, 200, and 500nM TNP-BSA. Regeneration was done by injection of 30 seconds of 60mM HCl. Curves were analyzed with BiaEvaluation 4.1 software (Biacore AB) according to the heterogenous ligand model, because of the polyclonal nature of the captured antibodies.
Bone marrow chimeras
Bone marrow (BM) recipients were lethally irradiated (2 × 5 Gy). Donor BM was isolated by flushing tibia and femurs. Recipient mice received 107 donor BM cells intravenously. Reconstitution efficiency was assessed by FACS analysis of blood leukocytes. Eight weeks after engraftment, mice were immunized intraperitoneally with 100 μg PE in alum.
PCR analysis of BCR clones and spectratyping
Total mRNA of pooled cells sorted to > 99% purity was extracted by Trizol method, after which cDNA was generated. BCR VH-CH elements were amplified by PCR, using VH186.2 primer (5′-GCTGTATCATGCTCTTCTTG-3′), Cγ1 primer (5′-GGATGACTCATCCCAGGGTCACCATGGAGT-3′, or the Cμ1 primer (5′-CATTTGGGAAGGACTGACTC-'3). For the analysis of IgG1+ cells, a nested PCR was performed using the VH186.2 primer (5′- GGTGTCCACTCCCAGGTCCA-3′) in combination with a FAM-labeled Cγ1 primer (5′-CCAGGGGCCAGTGGATAGAC-3′). Products were run through Genescan and analyzed with GeneMapper (ABI) for spectratyping. The PCR product was cloned in pGEM-T (Promega) and colonies with the VH186.2 fragment using Big Dye Terminator kit (Applied Biosystems). Sequences were analyzed using the International ImMunoGeneTics information system (http://imgt.cines.fr). Alignment and diversity analysis was performed with the ClustalW2 tool (http://www.ebi.ac.uk/Tools/clustalw2/). Divergence was determined by calculating the percentage of divergence between all pairs of sequence from a multiple alignment. Phylogenetic trees were then constructed by applying the Neighbor Joining Method18 to the distance matrix.
Results
Noxa is rapidly up-regulated in activated B cells
Purified murine B cells were stimulated with BCR agonistic antibodies in combination with LPS. Addition of IL-4 resulted in a phenotype resembling GC B cells isolated ex vivo (CD38dimGL7+, Figure 1A). A fraction of the cells indeed differentiated into IgG1-producing plasma cells (supplemental Figure 1A-B, data not shown). Although these cells are not actual GC B cells, we used these conditions as a model to study various ways of B-cell activation.
Noxa is induced in activated B cells. (A) Representative FACS plots of activated B cells. Gated is for B220+ cells. Top panels: B cells isolated directly ex vivo from (left) naive or (right) mice immunized 12 days prior with 50 ug of TNP-KLH (30:1) in alum. Bottom panels: in vitro cultured, purified B cells after 4 days of stimulation with anti-IgM and LPS in the (left) absence or (right) presence of 20 ng/mL IL-4. (B) Expression profiling by RT-MLPA of purified mouse B cells after in vitro stimulation. Cells were stimulated with anti-IgM/LPS alone (−IL4), or in combination with IL-4 (+IL4). Gene induction of pro and antiapoptotic molecules is represented in a heat-map after log2 transformation of expression levels (scale −2.5-2.5), relative to averaged values of unstimulated cells at day 0 (n = 3 per genotype. Shown is one of at least 3 experiments). (C) RT-PCR analysis for Noxa, Bim, Mcl-1, and 18S ribosomal RNA of anti-IgM/LPS (−IL4) and anti-IgM/LPS/IL-4 (+IL4) stimulated B cells. (D) Western blot analysis of total purified B cells after anti-IgM/LPS (−IL4) and anti-IgM/LPS/IL-4 (+IL4) stimulation. A representative result of 3 independent experiments is shown. (E) Expression profiling by RT-MLPA of cell sorted FB (B220+IgM+IgD+CD21/35+), MZB (B220+IgM+IgD−CD21/35bright), and GC (B220+CD38dimGL7bright) B cells of PE immunized mice, 12 days after immunization (n = 3 per genotype). Shown is 1 of 3 independent experiments. Mean and SEM are shown.
Noxa is induced in activated B cells. (A) Representative FACS plots of activated B cells. Gated is for B220+ cells. Top panels: B cells isolated directly ex vivo from (left) naive or (right) mice immunized 12 days prior with 50 ug of TNP-KLH (30:1) in alum. Bottom panels: in vitro cultured, purified B cells after 4 days of stimulation with anti-IgM and LPS in the (left) absence or (right) presence of 20 ng/mL IL-4. (B) Expression profiling by RT-MLPA of purified mouse B cells after in vitro stimulation. Cells were stimulated with anti-IgM/LPS alone (−IL4), or in combination with IL-4 (+IL4). Gene induction of pro and antiapoptotic molecules is represented in a heat-map after log2 transformation of expression levels (scale −2.5-2.5), relative to averaged values of unstimulated cells at day 0 (n = 3 per genotype. Shown is one of at least 3 experiments). (C) RT-PCR analysis for Noxa, Bim, Mcl-1, and 18S ribosomal RNA of anti-IgM/LPS (−IL4) and anti-IgM/LPS/IL-4 (+IL4) stimulated B cells. (D) Western blot analysis of total purified B cells after anti-IgM/LPS (−IL4) and anti-IgM/LPS/IL-4 (+IL4) stimulation. A representative result of 3 independent experiments is shown. (E) Expression profiling by RT-MLPA of cell sorted FB (B220+IgM+IgD+CD21/35+), MZB (B220+IgM+IgD−CD21/35bright), and GC (B220+CD38dimGL7bright) B cells of PE immunized mice, 12 days after immunization (n = 3 per genotype). Shown is 1 of 3 independent experiments. Mean and SEM are shown.
Expression of 40 apoptosis genes was analyzed over time by RT-MLPA.19 Direct NF-κB targets, such as Bcl-XL and A1,20 were rapidly induced after activation under both conditions (Figure 1B). Expression of Bcl-2 was reduced only after addition of IL-4, which corresponds with its regulation in GC B cells.3 Bmf was down-regulated in CD38dimGL7+ B cells, as previously described.21 Interestingly, of the BH3-only members, only Noxa was up-regulated in both presence and absence of IL-4 (Figure 1B). B cells from mice lacking p53 were still able to up-regulate Noxa, though to a lesser extent than wild-type (WT) cells (supplemental Figure 1C). Both Mcl-1 and Bim play an important role in mature B cells, yet their regulation is primarily on a protein level.22-26 In our experiments we found modest differential mRNA expression of Bim, and Mcl-1 was gradually down-regulated as determined by RT-MLPA and RT-PCR (Figure 1B-C). Mcl-1 protein levels quickly increased after activation, and Bim protein was induced from day 2 to 4 after stimulation (Figure 1D), as previously described.25
Next we investigated mRNA expression of apoptosis regulating genes in ex vivo isolated mature B cells. Mice were immunized with R-Phycoerythrin in alum and splenic GC B cells were sorted twelve days after injection. As a control, follicular B (FB) cells and marginal zone B (MZB) cells were isolated from naive WT mice. The gene transcription profile of MZB and GC B cells was compared with that of FB cells by RT-MLPA. Mcl-1 and Bim were not differentially regulated, whereas Bcl-XL and Noxa were up-regulated in GC B cells, compared with resting FB cells (Figure 1E). Noxa expression in GC B cells was lower than in vitro activated cells, suggesting that Noxa expression in vivo may be higher early after B-cell activation. In accordance with previous findings,27 Bcl-2 was higher in MZB cells, but down-regulated in the germinal center.
Thus, on B-cell activation, major changes occur in expression of apoptotic regulators. We chose to further investigate Noxa, because the ablation of Noxa alters the selection process of antigen-reactive T cells, and moreover conditional deletion of its antagonist Mcl-1 in B cells blocks GC formation and memory B-cell generation.17
Noxa regulates survival of activated B cells in vitro and in vivo
Noxa-deficient cells responded similarly to in vitro stimulation as WT cells and showed no difference in differentiation. When CD38+GL7+ cells were compared, no differences in survival were observed (Figure 2A, data not shown). However, in cultures stimulated with IL-4 we found consistent differences in the survival of CD38dimGL7+ B cells (Figure 2A-B). This fraction represents the most activated B-cell subset, as demonstrated by the high number of dividing cells compared with CD38+ B cells (Figure 2C, supplemental Figure 2A). No differences in proliferation were observed between WT and Noxa−/− for any fraction of B cells (Figure 2C, supplemental Figure 2A). The reduced number of apoptotic CD38dimGL7+ cells in cultures with Noxa−/− cells correlated with a significant increase in IgG1 secretion (Figure 2D). These data indicate that Noxa ablation augments survival of activated B cells in vitro and prompted us to analyze the effects of Noxa deficiency in vivo.
Noxa ablation provides a survival advantage for activated B cells in vitro. B cells were purified from mouse spleens and stimulated in vitro with anti-IgM/LPS alone (−IL4) or in combination with 20 ng/mL IL-4 (+IL4). Representative results from one of at least 4 independent experiments are shown. (A) Quantification of apoptotic cell numbers (% annexin V+/DNAdye+ cells) in the CD38+GL7+ and CD38dimGL7+ (latter are not present in cultures without IL4) populations is shown after 2 days of stimulation (n = 3 per genotype). (B) FACS plots of B220+CD38dimGL7+ cells stained with annexin V and DNA dye after 2 days of stimulation with anti-IgM/LPS and IL-4. (C) CFSE dilution of B220+CD38dimGL7+ cells after 2 days of stimulation with anti-IgM/LPS and IL-4. (D) Concentration of soluble IgG1 in the supernatant of cell cultures, determined by ELISA (n = 3 per genotype). Mean and SEM are shown (**P < .005; Student t test)
Noxa ablation provides a survival advantage for activated B cells in vitro. B cells were purified from mouse spleens and stimulated in vitro with anti-IgM/LPS alone (−IL4) or in combination with 20 ng/mL IL-4 (+IL4). Representative results from one of at least 4 independent experiments are shown. (A) Quantification of apoptotic cell numbers (% annexin V+/DNAdye+ cells) in the CD38+GL7+ and CD38dimGL7+ (latter are not present in cultures without IL4) populations is shown after 2 days of stimulation (n = 3 per genotype). (B) FACS plots of B220+CD38dimGL7+ cells stained with annexin V and DNA dye after 2 days of stimulation with anti-IgM/LPS and IL-4. (C) CFSE dilution of B220+CD38dimGL7+ cells after 2 days of stimulation with anti-IgM/LPS and IL-4. (D) Concentration of soluble IgG1 in the supernatant of cell cultures, determined by ELISA (n = 3 per genotype). Mean and SEM are shown (**P < .005; Student t test)
Under homeostatic conditions, phenotypic analyses did not reveal any changes in B cell numbers, phenotype or levels of BCR cell-surface expression in Noxa-deficient animals (supplemental Figure 2B-C). At 4 weeks of age, WT and Noxa−/− mice showed similar IgM and IgG levels in circulation (data not shown). However, at 12 weeks, Noxa−/− mice had modest hyper-gammaglobulinemia, most predominantly of the IgG1 and IgG2b subclasses, whereas levels of IgM antibodies were unaltered. (Figure 3A).
Noxa ablation provides a survival advantage for activated B cells in vivo. (A) The concentration of immunoglobulins in the serum of 12-week-old naive mice (n = 6 per genotype; 1 of 2 experiments is shown). (B-D) Mice were infected intranasally with Influenza A and analyzed 10 days after infection. (Bi) FACS staining of splenic B cells 10 days after infection. Gated is for B220+ cells. (ii) Absolute numbers of GC B cells in (top) the spleen and (bottom) the draining (mediastinal, mLN) lymph node was analyzed by FACS. (iii) Representative pictures (100× enlarged) of immunostained splenic sections. B220+ (yellow) and PNA+ (purple) cells were stained. (C) Mice were infected with influenza and after 10 days injected intraperitoneally with BrdU. Two hours after injection, BrdU incorporation was analyzed by FACS. Histograms of FB and GC B cells in the spleen, stained intracellularly for BrdU are shown (n = 5 per genotype). (D) Direct ex vivo quantification of cells with active caspase-3 in FB and GC B cells, 10 days after influenza infection. (i) FACS plots of splenic B cells stained for cells with active caspase-3 using the Vybrant FAM caspase-3 assay. Gated is for GC B cells (B220+CD38dimCD95+). (ii) Quantifications of B-cell subsets with active caspase-3 (n = 3-8 per genotype; 1 of 2 independent experiments with similar results is shown). Mean and SEM are shown (*P < 0,05, **P < .005; Student t test).
Noxa ablation provides a survival advantage for activated B cells in vivo. (A) The concentration of immunoglobulins in the serum of 12-week-old naive mice (n = 6 per genotype; 1 of 2 experiments is shown). (B-D) Mice were infected intranasally with Influenza A and analyzed 10 days after infection. (Bi) FACS staining of splenic B cells 10 days after infection. Gated is for B220+ cells. (ii) Absolute numbers of GC B cells in (top) the spleen and (bottom) the draining (mediastinal, mLN) lymph node was analyzed by FACS. (iii) Representative pictures (100× enlarged) of immunostained splenic sections. B220+ (yellow) and PNA+ (purple) cells were stained. (C) Mice were infected with influenza and after 10 days injected intraperitoneally with BrdU. Two hours after injection, BrdU incorporation was analyzed by FACS. Histograms of FB and GC B cells in the spleen, stained intracellularly for BrdU are shown (n = 5 per genotype). (D) Direct ex vivo quantification of cells with active caspase-3 in FB and GC B cells, 10 days after influenza infection. (i) FACS plots of splenic B cells stained for cells with active caspase-3 using the Vybrant FAM caspase-3 assay. Gated is for GC B cells (B220+CD38dimCD95+). (ii) Quantifications of B-cell subsets with active caspase-3 (n = 3-8 per genotype; 1 of 2 independent experiments with similar results is shown). Mean and SEM are shown (*P < 0,05, **P < .005; Student t test).
To test if the survival of B cells was affected during immune activation, mice were infected intranasally with the influenza virus A/PR8/34. Influenza causes a strong GC response, which reaches its peak 10 to 12 days after infection, after which it slowly declines (supplemental Figure 3B). Although Noxa-deficient animals had a short delay in recovery from infection, as measured by shifts in bodyweight (Wensveen et al16 and data not shown), no mice succumbed to the virus. Flow cytometry showed that Noxa-deficient mice formed more GC cells in the spleen and the mediastinal lymph nodes (mLN), which drain the lungs (Figure 3Bi-ii). These findings were corroborated by immunohistochemistry, which revealed enlarged germinal centers both in spleen and mLN 10 days after infection (Figure 3Biii, supplemental Figure 3A). The kinetics of the GC response indicated that Noxa-deficient animals have increased GC B cell numbers at the peak of the response, but not at early or late time points (supplemental Figure 3B).
We investigated whether increased proliferation or reduced apoptosis was responsible for this effect. Cell division, measured by intracellular staining for BrdU incorporation after a 2 hour BrdU pulse, and separately by the presence of the proliferation-associated antigen Ki67, was not different between GC B cells of influenza-infected WT and Noxa-deficient mice (Figure 3C, supplemental Figure 3C-D). However, when caspase-3 activity was assessed, a modest but significant reduction in the number of cells, which stained positive for this apoptosis marker was observed in germinal centers, but not follicular B cells especially of the spleens of Noxa−/− animals (Figure 3Di-ii). Because apoptotic cells are rapidly cleared by tangible body macrophages, these modest differences may well underestimate the actual in vivo situation. In summary, the in vitro and in vivo observations point to a nonredundant role of Noxa in the regulation of apoptosis preceding or during the GC reaction.
Ablation of Noxa results in low-affinity antibody responses
Next, WT and Noxa−/− mice were immunized with PE/Alum, and GC B cells were analyzed for their ability to bind PE. The peak of the GC response was on day 12 after immunization (data not shown). GC B cells of both WT and Noxa−/− mice bound PE to a higher extent than FB cells. Interestingly, Noxa-deficient animals showed a reduction in GC B cells that bound PE with high intensity (Figure 4A-B). This resulted in an overall reduction in the PE binding of GC B cells in these mice, as detected by reduced mean fluorescence intensity (MFI; Figure 4C). This decrease was especially apparent under limiting concentrations of PE, implying that high-affinity PE-specific B cells were specifically absent in Noxa−/− mice (supplemental Figure 4A). The observed effect could not be attributed to altered BCR expression, as the MFI of IgG1 expression was not different between WT and Noxa−/− mice, yet PE/IgG1 ratios were significantly reduced in Noxa−/− mice after PE-Immunization (supplemental Figure 4B-D).
Ablation of Noxa results in the generation of low-affinity antibody responses. (A-C) Mice were immunized with PE, and 12 days after immunization antigen-specific B cells were visualized by FACS staining (n = 5 per genotype). One of 2 independent experiments with similar results is shown. (A) FACS plots after ex vivo staining with 25 μg/mL PE. Arbitrary gating is applied to distinguish between PEbright and PE−/low cells. (B) Fraction of PEbright (gated as indicated in panel A) cells within GC cells. (C) Quantification of MFI data shown in panel A (stained with 25 μg/mL PE) shows significantly reduced PE-binding of Noxa−/− B cells. Normalization for differences in background staining between GC and FB cells was applied, based on signal within the PE− cells. This allows for direct comparison of PE staining-intensity of B-cell subsets. (D) Mice were immunized with TNP-KLH and TNP-specific antibody responses were assessed by ELISA under limiting serum dilutions with fixed TNP-BSA coating (5 μg/mL). Shown are IgM (left), IgG1 (middle), and IgG2b (right) responses (n = 5 per genotype; 1 of 2 independent experiments with similar results is shown). X-axis represents the percentage of antiserum in the dilution buffer used for detection of antibodies. (E) Assessment of the KD of TNP-specific IgG1 antibodies by biacore analysis 14 days after TNP-KLH immunization. (F) Mice were infected intranasally with Influenza A. Virus-specific IgM and IgG1 antibody responses were assessed after 2 weeks by ELISA under limiting antigen dilutions with fixed serum concentration (n = 8 per genotype; 1 of 2 independent experiments with similar results is shown). Serum of mice before infection was used as a negative control (triangles). In all panels mean and SEM are shown (*P < .05, **P < .005; Student t test).
Ablation of Noxa results in the generation of low-affinity antibody responses. (A-C) Mice were immunized with PE, and 12 days after immunization antigen-specific B cells were visualized by FACS staining (n = 5 per genotype). One of 2 independent experiments with similar results is shown. (A) FACS plots after ex vivo staining with 25 μg/mL PE. Arbitrary gating is applied to distinguish between PEbright and PE−/low cells. (B) Fraction of PEbright (gated as indicated in panel A) cells within GC cells. (C) Quantification of MFI data shown in panel A (stained with 25 μg/mL PE) shows significantly reduced PE-binding of Noxa−/− B cells. Normalization for differences in background staining between GC and FB cells was applied, based on signal within the PE− cells. This allows for direct comparison of PE staining-intensity of B-cell subsets. (D) Mice were immunized with TNP-KLH and TNP-specific antibody responses were assessed by ELISA under limiting serum dilutions with fixed TNP-BSA coating (5 μg/mL). Shown are IgM (left), IgG1 (middle), and IgG2b (right) responses (n = 5 per genotype; 1 of 2 independent experiments with similar results is shown). X-axis represents the percentage of antiserum in the dilution buffer used for detection of antibodies. (E) Assessment of the KD of TNP-specific IgG1 antibodies by biacore analysis 14 days after TNP-KLH immunization. (F) Mice were infected intranasally with Influenza A. Virus-specific IgM and IgG1 antibody responses were assessed after 2 weeks by ELISA under limiting antigen dilutions with fixed serum concentration (n = 8 per genotype; 1 of 2 independent experiments with similar results is shown). Serum of mice before infection was used as a negative control (triangles). In all panels mean and SEM are shown (*P < .05, **P < .005; Student t test).
The possible reduction of high-affinity B cells in Noxa-deficient animals prompted us to measure T cell–dependent antibody responses. Mice of 6 to 8 weeks of age were immunized with a single intraperitoneal injection of TNP-KLH in alum, and antibody production was assessed after 2 weeks. No differences in activation markers, such as MHC-II induction (supplemental Figure 5A) were observed. TNP-specific IgM levels were similar between Noxa−/− and WT mice, but a clear difference in antibody binding properties was observed for IgG1 and IgG2b (Figure 4D). This effect was not specific for TNP, as similar observations in antibody-binding properties were observed after PE immunization (supplemental Figure 5B). The decreased slope and lower plateau of the IgG1 and IgG2b binding curves indicated a lower affinity of the antibodies in Noxa-deficient mice. To confirm this notion, antibody affinity after TNP-KLH immunization was assessed using 2 other techniques. First, binding of antisera was assessed after titration of coated antigen. The binding curves from antibodies of Noxa-deficient animals of the IgG1, but not the IgM isotype rapidly declined when antigen-concentrations were reduced (supplemental Figure 5C), which is a good indication of reduced antigen-affinity. Next, average antigen affinity of the immune sera was measured by Biacore analysis. This revealed that TNP-specific antibodies from Noxa−/− mice had a significantly higher KD, indicating that these mice indeed generated antibody responses of reduced affinity/avidity (Figure 4E). The effects previously described did not depend on the adjuvant used, because similar results were obtained with TNP-KLH/IFA (data not shown).
To examine whether plasma cell numbers were affected in Noxa-deficient mice, TNP specific cells were quantified by enzyme-linked immunospot (ELISPOT; supplemental Figure 5D). Noxa−/− mice generated equal or even moderately increased numbers of antigen-specific IgG1-producing plasma cells, in agreement with increased GC cell numbers observed earlier (Figure 3B). Finally, to examine whether the observed B-cell phenotype in Noxa−/− mice also occurred after a physiologic virus infection, we measured humoral responses in influenza infected mice. Virus-specific IgG1-antibody responses were lower in Noxa−/− mice and the differences in the slopes of the IgG1 binding curves, both under conditions of limiting antiserum or limiting antigen, between WT and mutant mice again indicated a lower antibody affinity in Noxa−/− mice (Figure 4F, supplemental Figure 5E). The reduced affinity in Noxa-deficient animals persisted over time, because 50 days after infection similar observations were made (data not shown).
Together, these findings indicate that antigenic stimulation in the absence of Noxa results in the generation of low affinity IgG+ B cells and plasma cells, possibly as a result of persistent survival of low-affinity B-cell clones.
Reduced BCR affinity in Noxa−/− mice after antigen stimulation results from a B-cell intrinsic defect
The induction of Noxa in activated B cells suggests that the inability of Noxa−/− mice to generate high-affinity antibody responses is B-cell intrinsic. However, as Noxa deficiency also affects selection of high-affinity T cells,16 impaired T-cell help may also contribute to the observations in B-cell responses. To evaluate this, mixed BM chimeric mice were generated in WT (CD45.2) recipients. To ensure that all generated B cells received help from WT T cells, 80% of the injected cells was of WT (CD45.2) origin, whereas 20% of cells was derived from Noxa−/− (CD45.1) mice. Engraftment of BM after lethal irradiation of recipient mice was similar for WT and Noxa−/− cells, as 8 weeks after transfer, all measured immune-cell subsets consisted of 10%-20% of Noxa−/− cells (Figure 5A). Two months after transfer, mice were immunized with PE/Alum, and 12 days later antigen-specific cells were analyzed. Both WT and Noxa−/− cells were capable of generating PE-specific B cells. When the ratio of WT to Noxa−/− cells in the PE-positive population was compared with that in the FB cell compartment, a significant enrichment of Noxa-deficient cells was observed in the GC of immunized mice, which was more pronounced in the spleen than in the mLN (Figure 5B-C, supplemental Figure 6A-D). However, Noxa-deficient GC B cells showed on average significantly less PE binding, predominantly in the spleen (Figure 5B-D), which appeared to be the result of a reduced fraction of cells that bound PE with high intensity (supplemental Figure 6E-F). Absolute numbers of PEbright cells (defined by arbitrary gating) demonstrated a similar difference between WT and Noxa−/− cells, suggesting that the increased contribution of Noxa-deficient cells to the GC reaction is primarily the result of an increased number of low-affinity B cells.
Reduced BCR affinity in Noxa−/− mice after antigen stimulation results from a B cell intrinsic defect. WT (CD45.2) recipients (n = 10) were lethally irradiated and transferred with mixed WT (CD45.2) and Noxa−/− (CD45.1) BM in a 4:1 ratio. Two months after transfer, 8 mice were immunized with PE, and after 12 days B-cell responses were assessed. (A) Ratio of Noxa−/− cells in indicated lymphocyte populations before immunization in the blood of bone marrow chimeric mice 8 weeks after transfer. Presence of Noxa−/− CD45.1+ cells within B cells (B220+CD11b−), CD4 (CD4+CD3+) and CD8 (CD8+CD3+) T cells was quantified. (B) Histograms of FB cells and GC B cells in BM chimeric mice 12 days after PE/Alum immunization. Top panels: fraction of Noxa−/− cells (CD45.1+) within FB cells and GC B cells. Bottom panels: overlay of CD45.1+ and CD45.1− cells within the FB and GC B cell pools, stained with PE. (C) Quantification of the ratio of Noxa−/−/WT cells in the FB versus GC B cell fractions as shown in panel B. Noxa−/− cells are enriched within the antigen-specific pool. (D) Quantification of the average PE binding of GC B cells 12 days after immunization. MFI data shows significantly reduced PE-binding of antigen-specific Noxa−/− GC B cells. Mean and individual data points are shown (*P < .05, **P < .005; Student t test)
Reduced BCR affinity in Noxa−/− mice after antigen stimulation results from a B cell intrinsic defect. WT (CD45.2) recipients (n = 10) were lethally irradiated and transferred with mixed WT (CD45.2) and Noxa−/− (CD45.1) BM in a 4:1 ratio. Two months after transfer, 8 mice were immunized with PE, and after 12 days B-cell responses were assessed. (A) Ratio of Noxa−/− cells in indicated lymphocyte populations before immunization in the blood of bone marrow chimeric mice 8 weeks after transfer. Presence of Noxa−/− CD45.1+ cells within B cells (B220+CD11b−), CD4 (CD4+CD3+) and CD8 (CD8+CD3+) T cells was quantified. (B) Histograms of FB cells and GC B cells in BM chimeric mice 12 days after PE/Alum immunization. Top panels: fraction of Noxa−/− cells (CD45.1+) within FB cells and GC B cells. Bottom panels: overlay of CD45.1+ and CD45.1− cells within the FB and GC B cell pools, stained with PE. (C) Quantification of the ratio of Noxa−/−/WT cells in the FB versus GC B cell fractions as shown in panel B. Noxa−/− cells are enriched within the antigen-specific pool. (D) Quantification of the average PE binding of GC B cells 12 days after immunization. MFI data shows significantly reduced PE-binding of antigen-specific Noxa−/− GC B cells. Mean and individual data points are shown (*P < .05, **P < .005; Student t test)
Together, these data indicate that activated Noxa-deficient low-affinity B cells have an intrinsic competitive advantage over WT cells, which is independent of T-cell help.
Noxa ablation results in increased diversity of B-cell clones after immunization
So far, our data indicate that Noxa deficiency leads to decreased affinity of the overall B-cell pool, probably via increased survival of low affinity cells. This may be the result of decreased affinity maturation in the GC, similar to what is observed in Bim-deficient animals.2 However, as Cre-mediated deficiency of Mcl-1 in activated B cells does not lead to altered hypermutation rates,17 the Noxa/Mcl-1 axis might play a separate role in the selection of antigen-specific B-cell clones.
To investigate the role of Noxa in affinity maturation, an established model to study hypermutation rates was applied. When C57BL/6 mice are immunized with nitrophenylacetyl linked to chicken γglobulin (NP-CGG), GC cells primarily use the VH186.2 gene and a large fraction of these cells displays an affinity-enhancing (W33L) mutation.28 When Noxa-deficient mice were immunized with NP-CGG, no difference in the average number of mutations per sequence was observed compared with WT animals. (supplemental Figure 7A-B, WT = 7,9 ± 1.0, Noxa−/− = 6,8 ± 3.5). Importantly, a similar frequency of W33L mutations was found in WT and Noxa−/− mice, again indicating that affinity maturation is not greatly affected in Noxa−/− mice (supplemental Figure 7A). On the other hand, the number of GC-seeding clones, as determined by quantification of clones with identical CDR3 regions, was doubled in Noxa-deficient animals (supplemental Figure 7C). This suggests a role for Noxa in the control of the number of activated B-cell clones, rather than of affinity maturation.
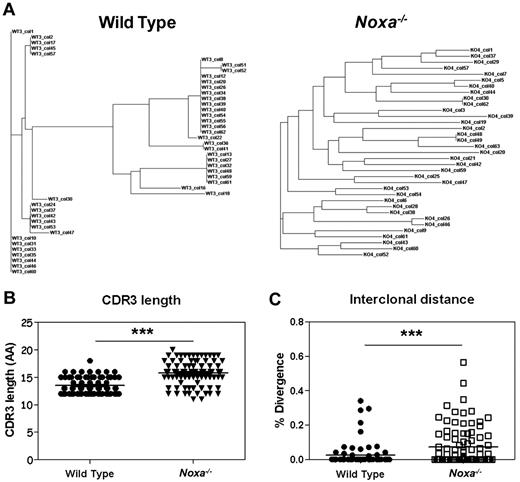
To test this premise with another antigen, clonal analysis of antigen-specific B cells was performed after PE-immunization. Spectratype analysis on naive IgM-positive FB cells revealed a normal Gaussian distribution with no increase of exceptionally short or long sequences within the CDR3 regions of the VH186.2 genes of Noxa−/− mice (supplemental Figure 8A) which argues against major differences within the naive compartment of these animals. At the peak of the GC response after PE/Alum immunization, antigen-specific B cells were isolated and analyzed for clonal diversity within the VH186.229 family (supplemental Figure 8B for spectratyping). It has been estimated that germinal centers are seeded by 40 to 50 precursors,30 of which only a fraction forms progeny that significantly contributes to the plasma-cell population.8 When the frequency of different clones based on their CDR3 region was compared between WT and Noxa−/− cells, we observed that, as after NP-CGG immunization, Noxa−/− mice showed many different clones (Figure 6A, supplemental Figure 8C). Analysis of sequence similarity revealed a significant increase in diversity between antigen-specific clones in Noxa−/− mice, with a less restricted CDR3 length (Figure 6B-C). Phylogenetic analysis showed 3 to 6 clusters per WT mouse, whereas Noxa−/− mice harbored on average 12 of these clusters. Again, no significant differences in hypermutation frequencies were found (data not shown).
Noxa ablation results in survival of multiple B cell clones after immunization. Mice were immunized with PE and after 12 days, antigen-specific germinal center B cells were sorted and analyzed for clonal diversity. Pooled data of 3 mice per genotype from 2 independent experiments were used. (A) Representative phylogenetic trees of antigen-specific cells from a WT (left) and Noxa−/− mouse (right) shows increased clonal diversity within the latter. (B) CDR3 lengths of PE specific clones (AA indicates amino acids). (C) Interclonal diversity as a percentage of divergence between all pairs of sequences from a multiple alignment (WT n = 73, Noxa−/− n = 93; ***P < .0001; Student t test and Mann-Whitney test).
Noxa ablation results in survival of multiple B cell clones after immunization. Mice were immunized with PE and after 12 days, antigen-specific germinal center B cells were sorted and analyzed for clonal diversity. Pooled data of 3 mice per genotype from 2 independent experiments were used. (A) Representative phylogenetic trees of antigen-specific cells from a WT (left) and Noxa−/− mouse (right) shows increased clonal diversity within the latter. (B) CDR3 lengths of PE specific clones (AA indicates amino acids). (C) Interclonal diversity as a percentage of divergence between all pairs of sequences from a multiple alignment (WT n = 73, Noxa−/− n = 93; ***P < .0001; Student t test and Mann-Whitney test).
Together, these data indicate that Noxa is involved in the elimination of low-affinity clones by setting a threshold for survival. Ablation of the Noxa gene leads to increased numbers of clones that participate in the GC reaction with unaffected hypermutation rates, which ultimately results in antibody responses of reduced affinity.
Booster immunization cannot overcome the inability of Noxa−/− mice to mount high affinity antibody responses
In vaccination strategies, humoral immune responses can be improved by repeated administration of antigen (boosting), which increases the quantity and quality of the specific antibodies; a process that may include further maturation of memory B cells in GCs.31 Therefore, we tested whether the observed defect in Noxa−/− mice to preferentially form low-affinity antibodies can be overcome by boosting.
Noxa-deficient animals were immunized with TNP-KLH/Alum and boosted after 28 days. At several time points, TNP-specific antibody levels were determined. Again, TNP-specific IgM levels were similar between Noxa−/− and WT mice, but after secondary immunization, IgG1 and especially IgG2b levels were significantly reduced in Noxa−/− sera compared with WT (supplemental Figure 9). When analyzed at day 7 after boosting under limiting antigen concentrations, the dilution curves in ELISA for IgG1 and IgG2b in Noxa−/− sera showed a clearly diminished slope and plateau, indicative of a reduced affinity of serum antibodies in Noxa−/− compared with WT animals (Figure 7A).
Ablation of Noxa results in memory B-cell responses of reduced quality and quantity. Mice were immunized with TNP-KLH and boosted after 28 days. Seven days after boost, antibody responses were analyzed. (n = 5 per genotype; 1 of 2 experiments with similar results is shown). (A) TNP-specific antibody responses were assessed by ELISA under limiting serum dilutions with fixed TNP-BSA coating (5 ug/mL). Shown are IgM (top), IgG1 (middle), and IgG2b (bottom) responses (WT indicates wild-type; and p.i.-serum, pre-immune serum, sera isolated from mice before immunization). (B) TNP-specific IgG1 antibody responses were assessed by ELISA under limiting TNP-BSA coating, using high (15:1, top panel) and low (4:1, bottom panel) avidity antigen. Results are expressed as percentage of the response at maximum antigen levels. (C) Numbers of TNP-specific IgG1 producing plasma cells in the spleen, measured by ELISPOT. Mean and SEM are shown (*P < .05, **P < .005, and ***P < .0001; Student t test).
Ablation of Noxa results in memory B-cell responses of reduced quality and quantity. Mice were immunized with TNP-KLH and boosted after 28 days. Seven days after boost, antibody responses were analyzed. (n = 5 per genotype; 1 of 2 experiments with similar results is shown). (A) TNP-specific antibody responses were assessed by ELISA under limiting serum dilutions with fixed TNP-BSA coating (5 ug/mL). Shown are IgM (top), IgG1 (middle), and IgG2b (bottom) responses (WT indicates wild-type; and p.i.-serum, pre-immune serum, sera isolated from mice before immunization). (B) TNP-specific IgG1 antibody responses were assessed by ELISA under limiting TNP-BSA coating, using high (15:1, top panel) and low (4:1, bottom panel) avidity antigen. Results are expressed as percentage of the response at maximum antigen levels. (C) Numbers of TNP-specific IgG1 producing plasma cells in the spleen, measured by ELISPOT. Mean and SEM are shown (*P < .05, **P < .005, and ***P < .0001; Student t test).
To corroborate these findings, antigens of variable avidity using high (15) and low (4) numbers of hapten per carrier protein were used in an ELISA binding assay. Under limiting concentrations of high-avidity antigen, Noxa−/− IgG1 antibodies showed reduced binding compared with those generated in WT mice (Figure 7B top panel). This became even more pronounced under limiting concentrations of low-avidity antigen. Under these conditions, differences between antibody dissociation constants were significantly increased (Figure 7B bottom panel), and showed approximately a 12-fold increase in KD for antibodies from Noxa-deficient mice (as calculated by curve-fitting). Antigen-specific plasma cell numbers were again not reduced, showing that there is no defect in differentiation, but in the amount of high-affinity antibodies produced (Figure 7C).
In summary, these data indicate that the differences in affinity cannot be overcome by boosting, but rather increase between WT and Noxa−/− mice after a second encounter of antigen.
Discussion
It is well established that apoptosis plays an essential role in the formation of high-affinity B-cell responses by executing cells that have been negatively selected.32-34 Much attention has been given to the role of apoptosis during the GC reaction.2-4 However, the molecular selection mechanisms that determine early phases of antigen-driven B-cell activation and selection for GC entry have largely remained unclear. We now show that apoptosis involving Noxa sets an important threshold for expanding B cells, which is distinct from hypermutation-driven affinity maturation. Ablation of Noxa results in an increased number of clones that are allowed to form plasmablasts, and this negatively affects the affinity of the ensuing pool of antibodies. Absence of Noxa does not completely block formation of high affinity antibodies, but strongly shifts the spectrum to overall lower affinity. Importantly, repeated immunization failed to correct this deficit.
As of yet it is unclear how exactly BCR affinity controls the balance between pro and antiapoptotic molecules, but a plausible parallel can be drawn between B cells and T cells. Similar to B cells, activated T cells up-regulate both Noxa and its antagonist Mcl-1.15 Targeted ablation of Mcl-1 results in the complete elimination of T and B cells, demonstrating its importance in lymphocyte survival.35 Recently, we and others have found that T cells specifically up-regulate the IL-2Rα subunit (CD25) on antigen encounter, which after binding IL-2, increases the stability of Mcl-1.16,36 In T cells, antigen triggering thus regulates sensitivity to apoptotic stimuli via indirect modulation of the levels of Bcl-2 members. In B cells, Mcl-1 has recently been demonstrated to be essential for the survival of activated B cells,17 even though the underlying mechanism for the dependence of this protein on activation remained unclear. Based on our data, we propose that also in B cells Noxa, in conjunction with its antagonist(s) Mcl-1 and/or A137 controls the number of clones contributing to an immune response. Alternatively, affinity-based survival may be controlled via Bim, of which the binding spectrum of Bcl-2 members overlaps with Noxa.38 Bim protein stability in activated B cells is tightly controlled by the prosurvival cytokines BAFF and APRIL.25 Binding of these proteins to their receptor leads to ERK-mediated Bim phosphorylation and subsequent degradation. Deficiency of BAFF, APRIL, or their receptors thus leads to greatly impaired B-cell responses in a variety of models,39,40 which can be overcome by overexpression of Bcl-2.41 Ablation of Bim leads to increased GC cell numbers and reduced selection by hypermutation.2 Noxa plays a role in the phase of B-cell selection, which precedes hypermutation-driven affinity maturation and limits the amount of clones that participate in B-cell responses, but by itself is not sufficient to induce cell death.38 To our knowledge, the role of Bim has not been studied in this phase of B cell selection. However, ERK-mediated degradation of Bim appears to be a likely mechanism for activated B cells to overcome the survival threshold set by transcriptional up-regulation of Noxa on BCR ligation. Therefore, we propose that apoptosis mediated by Noxa and Bim controls the number of clones that are allowed to enter the germinal center, whereas Bim and FAS2,42 contribute to affinity selection based on hypermutation (supplemental Figure 10).
It should be noted that it cannot be formally excluded, that Noxa-deficient mice on a clonal level have a naive compartment of increased diversity compared with WT mice. However, normal numbers of B-cell precursors and their phenotypic composition in Noxa-deficient animals, low Noxa expression in B-cell precursors (data not shown), a normal Gaussian division of VH-chain lengths within naive peripheral B cells, and a lack of auto-immunity in aged Noxa-deficient mice16 indicate that this is unlikely.
From the perspective of vaccine design, an important finding of our study is that Noxa does not only affect primary B-cell responses, but also greatly impairs recall responses. Interestingly, low BCR affinity has been associated with increased memory B-cell formation,10,43 which would imply increased memory cell numbers in Noxa−/− mice. Our preliminary findings indicate that this is indeed the case. It seems counterintuitive that low-affinity cells should contribute to memory. On reinfection with identical pathogens, clearance is primarily executed by high-affinity antibodies.44 Thus, to reestablish itself in a host, there will be selection for pathogens that have acquired mutations to avoid recognition by neutralizing antibodies. By sustaining a memory population of increased divergence, the likelihood of recognition of these mutated pathogens is increased. Although Noxa−/− mice perform less well after boosting with an identical antigen, it will be interesting to investigate the responsiveness against similar, but nonidentical antigens. Human vaccination strategies generally aim to induce a high-affinity antibody response that allows clearance of the targeted pathogen before disease development can occur. On the other hand, it might be desirable to elicit a broadly neutralizing response, capable of targeting the original pathogen, as well as its escape mutants. Identification and future manipulation of factors such as Noxa that may affect this aspect of an immune response may therefore be applicable in vaccine design.
In conclusion, we identify apoptosis regulated by Noxa as a mechanism to eliminate activated low-affinity B-cell clones during interclonal competition. This selection step reduces the number of B cells that participate in an immune response, and allow enhanced expansion of high-affinity cells during both primary and recall responses. We propose the Noxa/Mcl-1 axis as a promising therapeutic target for the manipulation of the broadness of antibody responses after vaccination.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Arnoud Marquart of the Deptartment of Vascular Medicine for Biacore Measurements, Marco Breuer and Tatjana Westphal (Netherlands Cancer Institute) for the p53−/− tissues, and Stefano Casola (the Fondazione Italiana per la Ricerca sul Cancro Institute of Molecular Oncology Foundation, Milan, Italy) for critical reading and helpful comments.
This work was sponsored by an Academisch Medisch Centrum PhD grant (F.M.W. and E.E.), an NWO-VIDI grant (A.M.d.B. and M.A.N.), and a Nederlandse Organisatie voor Wetenschappelijk Onderzoek VICI grant (K.P.J.M.v.G. and R.A.W.v.L.).
Authorship
Contribution: F.M.W. designed and performed experiments, analyzed data, and wrote the paper; K.P.J.M.v.G. and I.A.M.D. designed and performed experiments, analyzed data, and corrected the paper; A.M.d.B. performed experiments; H.Y. codeveloped and tested the murine MLPA probes; J.C.M.M. and M.A.N. designed and analyzed experiments; and E.E. and R.A.W.v.L. designed experiments, analyzed data, wrote the paper, and supervised the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Eric Eldering, Deptartment of Experimental Immunology, Academic Medical Center, Meibergdreef 9, 1105AZ Amsterdam, The Netherlands; e-mail: e.eldering@amc.uva.nl.
References
Author notes
I.A.M.D. and K.P.J.M.v.G share second authorship.
E.E. and R.A.W.v.L. share senior authorship







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal