Abstract
Lewis Y Ag (LeY) is a cell-surface tetrasaccharide that participates in angiogenesis. Recently, we demonstrated that LeY is a specific ligand of the recombinant lectin-like domain of thrombomodulin (TM). However, the biologic function of interaction between LeY and TM in endothelial cells has never been investigated. Therefore, the role of LeY in tube formation and the role of the recombinant lectin-like domain of TM—TM domain 1 (rTMD1)—in antiangiogenesis were investigated. The recombinant TM ectodomain exhibited lower angiogenic activity than did the recombinant TM domains 2 and 3. rTMD1 interacted with soluble LeY and membrane-bound LeY and inhibited soluble LeY-mediated chemotaxis of endothelial cells. LeY was highly expressed on membrane ruffles and protrusions during tube formation on Matrigel. Blockade of LeY with rTMD1 or Ab against LeY inhibited endothelial tube formation in vitro. Epidermal growth factor (EGF) receptor in HUVECs was LeY modified. rTMD1 inhibited EGF receptor signaling, chemotaxis, and tube formation in vitro, and EGF-mediated angiogenesis and tumor angiogenesis in vivo. We concluded that LeY is involved in vascular endothelial tube formation and rTMD1 inhibits angiogenesis via interaction with LeY. Administration of rTMD1 or recombinant adeno-associated virus vector carrying TMD1 could be a promising antiangiogenesis strategy.
Introduction
Angiogenesis is involved in physiologic processes such as embryogenesis, wound healing, and the female reproductive cycle, and also contributes to the pathogenesis of numerous disorders, including atherosclerosis, ischemic disease, arthritis, and cancer.1 Normally, angiogenesis is strictly controlled by the balance between angiogenic promoters and inhibitors.
Thrombomodulin (TM) is a type I–glycosylated membrane protein composed of 5 distinct domains. At the NH2-terminal is a C-type lectin-like domain followed by 6 consecutively repeated epidermal growth factor (EGF)–like domains and an O-glycosylation site-rich domain. Connected to these extracellular domains is a transmembrane domain followed by a short cytoplasmic tail.2 TM functions as part of the anticoagulant pathway; it binds thrombin on the cell surface and catalyzes proteolytic activation of protein C.2 Soluble TM fragments have been detected in the medium of cultured TM-expressing cells3 and human plasma and urine,4-6 but the physiologic significance of the soluble TM fragments remains unclear. Our previous study showed that the recombinant EGF-like domain plus the O-glycosylation site-rich domain of TM (rTMD23) promotes angiogenesis in vitro and in vivo.7 The multiple mechanisms underlying the anti-inflammatory activity of the recombinant lectin-like domain of TM have been demonstrated recently.8-11
Lewis Y Ag (LeY) belongs to the type II Lewis Ag family, which is structurally related to the determinants of the ABH blood group system. LeY is expressed on the cell membrane along with phospholipids,12 EGF receptors,13 and mucins,14 and its soluble form is up-regulated in the synovial fluid of human angiogenic rheumatoid arthritis.15 LeY has been suggested to be a participant in cell-cell interaction,16 arthritis,15 and cancer.17 In addition, ablation in endothelial cells of fucosyltransferase I, which is crucial for generation of the precursor of type II Lewis Ag, resulted in defective tube formation, suggesting the role of LeY in endothelial cell-cell contacts.18
Previously, we demonstrated that recombinant lectin-like domain of TM (rTMD1) suppressed lipopolysaccharide-induced inflammation through interaction with LeY-conjugated lipopolysaccharide.11 However, the biologic significance of the interaction between rTMD1 and LeY on the endothelial cell surface has never been investigated. In this study, the biologic function of LeY on endothelial cells and the antiangiogenic function of rTMD1 were investigated. In addition, recombinant adeno-associated virus (AAV) expression vector carrying TMD1 (AAV-TMD1) resulted in efficient suppression of angiogenesis with a single injection of AAV in mice.
Methods
Expression, purification, and characterization of rTMD proteins and the AAV expression system
The human TM cDNA was cloned from HUVECs by RT-PCR.19 TMD sequences, including recombinant TM ectodomain (rTMD123), rTMD23, and rTMD1, were subcloned into a Pichia pastoris protein expression vector, pPICZαA (Invitrogen), for use in yeast, and into a mammalian expression vector, modified pCR3 (Invitrogen), for use in human embryonic kidney 293 (HEK293) cells. The 6-His tag and c-Myc epitope in the C-terminus of recombinant TM domain (rTMD) proteins were used for purification and protein detection. The C-terminal tags of rTMD proteins were removed by enterokinase as described previously.11 The tag-removed rTMD1 (rTMD1Δ) was used to confirm the biologic function of rTMD1. Fermentation medium containing expressed rTMD protein was purified by a nickel-chelating Sepharose column (Amersham Pharmacia Biotech AB). The purified rTMD proteins were analyzed by SDS-PAGE and Western blotting, and identified with mass spectrometry analysis (Applied Biosystems). Amino acid sequence determinations were performed automatically by Edman degradation with a model 477A sequencer (Applied Biosystems). The concentration of rTMD protein was determined using the BCA kit (Pierce Biotechnology) with BSA as the standard. The assay for thrombin-dependent activation of protein C by rTMD proteins was performed as previously described.7 To generate AAV-TMD1 and AAV-luciferase (AAV-Luc) expression vectors, the genomic sequence of TMD1 and luciferase were subcloned into AAV serotype 2/8 vectors and were produced at the Research Vector Core, Harvard Gene Therapy Initiative (Harvard Medical School, Boston, MA). Briefly, the vectors are generated by tripartite transfection (AAV vector plasmid encoding the gene of interest, AAV helper plasmid pLT-RC08 encoding AAV Rep proteins from serotype 2 and Cap proteins from serotype 8, and adenovirus helper miniplasmid pHGTI-Adeno1) of 293A cells. The transfection was performed using a protocol developed by Xiao et al.20 Two days after transfection, the transfected cells were harvested, and lysed by repeated freeze-thaw cycles. After initial clearing of cell debris, the nucleic acid component of the virus producer cells was removed by benzonase treatment. The recombinant AAV vector particles were purified by iodixanol density gradient and Q-Sepharose column chromatography. The purified virus was dialyzed extensively against Dulbecco PBS with Mg and Ca (DPBS), concentrated by Amicon spin column, and titered by dot-blot hybridization. For in vivo studies, mice were given 1011 genome copies of AAV expression vectors in 100 μL of DPBS by IV injection. The expression of AAV-Luc was detected by a luciferase assay (Promega) of tissue extracts, and the expression of TMD1 was detected by RT-PCR with specific primers for TMD1 (5′-GCACGACTGCTTCGCGCTCTAC-3′ and 5′-TCGCACTGCTGCTCCTCCCAGA-3′); TM (Thbd, 5′-CTTGTGCAATAGGAGCACGA-3′ and 5′-GACACAAAAATGCTCGCAGA-3′); and Gapdh (5′-CCACACATGGTAGATACAAAGGAA-3′ and 5′-GCCAGGCGGCAGGTCAGGTCCACA-3′). In brief, mRNA was isolated from the liver with TRIzol reagent (Invitrogen), and the RT-PCRs were performed in an ABI PCR system 9700 (Applied Biosystems) for 3 minutes at 94°C followed by 25 cycles of amplification for 30 seconds at 94°C, 30 seconds at 60°C, and 30 seconds at 72°C, ending with one step for 5 minutes at 72°C. The expected amplicons of TMD1, Thbd, and Gapdh are 381, 130, and 601 bp, respectively.
Cell cultures
HUVECs were isolated by treatment with collagenase and cultured with medium 199 (M199) containing endothelial cell growth supplement (Millipore) and 16% FBS as previously described.7 Cells from the second to fourth passages were used in all of the experiments.
Cell proliferation assay
To evaluate the effect of rTMD proteins on DNA synthesis in HUVECs, a labeling kit (Roche Diagnostics GmbH) was used to assay BrdU incorporation according to the manufacturer's protocol.
Cell migration assay
The assay was performed in a 48-multiwell Boyden chamber (Neuro Probe) with 8-μm pore-size polycarbonate filters (Neuro Probe) coated with 0.1% gelatin (Sigma-Aldrich). EGF (R&D Systems), polyacrylamide-based multivalent Lewis X Ag, polyacrylamide-based multivalent LeY (LeY-PAA; Glycotech), rTMD123, or rTMD23 was added to the bottom compartment as a chemoattractant, and the migrated cells were stained with Liu stain and counted. In the analysis of inhibition by rTMD1, HUVECs were mixed with rTMD1 in the upper compartment, and chemoattractants were added to the bottom compartment. In other experiments, LeY-PAA was incubated with rTMD1 for 30 minutes before performing the experiment. The concentration of LeY-PAA (multivalent LeY-PAA, 30 kDa) was calculated according to the manufacturer's instructions.
Signal transduction assay and immunoprecipitation
Subconfluent HUVECs were starved in serum-free M199 for 4 hours, incubated with rTMD1 for 30 minutes, and finally stimulated with EGF (10 ng/mL) for 10 minutes. EGF receptor (EGFR) activation was evaluated by immunoprecipitating with a mAb against EGFR (LA-1; Upstate-Millipore), and detected by a rabbit polyclonal Ab against phosphorylated EGFR (Tyr-1173; Cell Signaling Technology) and a rabbit polyclonal Ab against EGFR (Cell Signaling Technology). The downstream effector of the EGFR was also analyzed with Abs against phosphorylated Akt (Ser-473), and Akt (Santa Cruz Biotechnology), respectively. For detection of LeY-modified EGFR and ICAM2, a lysate of HUVECs was immunoprecipitated with Ab against EGFR (sc-03; Santa Cruz Biotechnology) or ICAM2 (sc-23935; Santa Cruz Biotechnology) and then immunoblotted with Abs against LeY (ab3359; Abcam); normal mouse IgG was used as a negative control.
In vitro vascular tube formation assay
To evaluate the effect of LeY on endothelial tube formation in vitro, 15-well μ-slides (ibidi GmbH) were coated with 10 μL of Matrigel (BD Biosciences). HUVECs preincubated with rTMD1 or LeY Ab (ab23911; Abcam) 30 minutes were added to the wells in M199 containing 5% FBS and 3.75 μg/mL endothelial cell growth supplement. To analyze the effect of rTMD1 on EGF-mediated endothelial tube formation in vitro, HUVECs were starved for 12 hours in M199 containing 1% FBS, preincubated with rTMD1 30 minutes, and finally with EGF. The total tube length was measured using MetaMorph software (Molecular Device) and compared with the control at the indicated times. The micrographs of tube formation were taken and processed by an inverted microscope (Leica DM IRE2) equipped with a N PLAN 10×/0.25 objective lens and a CCD camera (CoolSNAP fx; Photometrics) using MetaMorph software at 25°C.
Labeling of rTMD1
To study the interaction of rTMD1 and LeY during endothelial tube formation on Matrigel, FITC-conjugated rTMD1 (rTMD1-FITC) or FITC-conjugated BSA (BSA-FITC) was prepared according to the procedure of Molecular Probes (F6434).
Immunofluorescence and confocal microscopy
Confluent HUVECs grown on 0.1% gelatin-coated cover glasses were fixed with 4% paraformaldehyde in PBS for 15 minutes, incubated with a mAb to LeY (ab3359; Abcam) with mouse normal IgM as control, and then with Alexa Fluor 546–conjugated goat secondary Abs (Invitrogen). For visualizing the interaction of rTMD1 with LeY on forming endothelial tubes, 0.5μM rTMD1-FITC or BSA-FITC was added to the cells. The images were captured using a Leica TCS SP2 confocal microscope equipped with a PLAN APO 63×/1.32 oil objective lens and an Olympus FV1000 confocal microscope equipped with a UPLANSAPO 60×/1.35 oil objective lens at 25°C. Three-dimensional reconstruction of the confocal z-stacks and the measurement of colocalization were performed using the MetaMorph program.
SPR assay
The surface plasmon resonance (SPR) assay was performed as previously described.11 Binding parameters for the interaction of rTMD1 or rTMD23 with LeY were measured using a BIAcore 3000 SPR-based biosensor system. The chip was activated with 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide/N-hydroxysuccinimide and then blocked with ethanolamine. Using the amine coupling protocol, the rTMD1Δ or tag-removed rTMD23 (rTMD23Δ) molecules were immobilized onto a CM chip (Biacore; GE Healthcare) at a concentration of 2200 response units. Multivalent LeY at various concentrations in PBS was passed through the flow cells at a rate of 20 μL/minute. All data were corrected for the response obtained using a blank reference flow cell. Binding data were evaluated using the BIAevaluation program Version 3.1 (Biacore).
Murine angiogenesis assay
To assess angiogenic effects in vivo, growth factor–reduced liquid Matrigel (0.5 mL) containing heparin (40 U/mL) with various combinations of EGF and rTMD1 was subcutaneously injected into FVB mice near the abdominal midline. Four days after implantation, mice were euthanized and the Matrigel plugs were surgically removed, photographed with a CMOS camera (600D, Canon), and the hemoglobin content of each Matrigel plug was measured with Drabkin reagent 525 (Sigma-Aldrich). To test different methods of administration of rTMD1, a protocol was designed. In brief, the mice were IV injected with AAV-TMD1 (1011 genome copies), AAV-Luc (1011 genome copies), or vehicle control (DPBS) 4 days before the implantation of EGF and heparin-containing Matrigel. One group of mice was subcutaneously injected with rTMD1 (200 μL of 80 μg/mL rTMD1/20 g mouse weight/d) daily for 4 days after the implantation of EGF and heparin-containing Matrigel.
Rat corneal micropocket assay
A rat corneal micropocket assay was performed as described previously.21,22 Uniformly sized pellets of Hydron (polyhydroxyethylmethacrylate [poly HEMA]; Sigma-Aldrich) containing various amounts of rTMD1 or 200 ng of EGF and sucralfate (Sigma-Aldrich) were made, and sex-matched 6- to 8-week-old Sprague-Dawley rats were used. After proper systemic anesthesia and subsequent application of a topical anesthetic and mydriatic to the eye, a micropocket in the cornea was generated 2 mm away from the limbus vessel at the temporal site of the eye. Neovascularization was observed 7 days after implantation under a stereomicroscope equipped with a CMOS camera (600D, Canon). The angiogenic index was calculated using the following formula: vessel area = 0.02π × clock hours × vessel length.23
Effect of rTMD1 on xenografts of Lewis lung carcinoma cells
To assess the effect of rTMD1 on tumor angiogenesis, C57BL/6 mice were given subcutaneous dorsal injections of 1 × 106 Lewis lung carcinoma cells. The mice were given IP injections of various amounts of Pichia-expressed rTMD1 (0.08 or 0.8 mg/kg body weight) once daily for 2 weeks. Tumors were harvested, photographed with a CMOS camera (600D, Canon) after 2 weeks, and cryosectioned. Intratumoral microvessels were detected using IHC by using an Ab against CD31 (BD Biosciences) and the images were captured using a Leica TCS SP2 confocal microscope equipped with a PLAN APO 40×/1.25 oil objective lens at 25°C. Intratumoral microvessel density was quantified as previously described.24 Briefly, microvessel density was calculated in each section of the tumor at 40× magnification by using the MetaMorph software.
Animal care
Animal care conditions and design of experiments were approved by the Institutional Animal Care and Use Committee of the National Cheng Kung University (Tainan, Taiwan).
Statistical analysis
Data are expressed as mean ± SD. Differences between more than 2 groups were compared by 1-way ANOVA followed by a Bonferroni multiple comparison test. Values of P < .05 were considered statistically significant.
Results
Expression, purification, and characterization of rTMD proteins
The rTMD proteins, rTMD123 (Ala1-Ser497), rTMD23 (Ala224-Ser497), and rTMD1 (Ala1-Ala155), were prepared by using a yeast protein expression system. To further confirm the function of rTMD1, rTMD1 was also prepared by using HEK293 cells. Calculated with the Mw tool of the ExPASy proteomic server (http://www.expasy.org), the molecular masses of rTMD123, rTMD23, and rTMD1, are 55.9, 32.8, and 20 kDa, respectively. The relative molecular masses of rTMD123, rTMD23, and rTMD1 expressed by the yeast expression system as determined by SDS-PAGE were ∼ 95, 63, and 35 kDa, respectively (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), because of modification by glycosylation. As previously shown,11 the molecular mass of rTMD1 expressed by the mammalian expression system was similar to that from the yeast expression system (supplemental Figure 1). Both rTMD123 and rTMD23 induced thrombin-dependent protein C activation whereas rTMD1 had no such activity, as expected. The analyses from mass spectrometry and NH2-terminal sequencing showed that rTMD proteins had the starting sequence at the fusion peptide, Glu-Phe, followed by the expected sequence of rTMD. The rTMD1 proteins obtained from either the yeast or the mammalian protein expression system possessed similar biologic activity in various assay systems, including proliferation, migration, tube formation, signaling, Matrigel, and SPR analysis.
Effects of rTMD proteins on proliferation and migration of HUVECs as well as on angiogenesis in vitro and in vivo
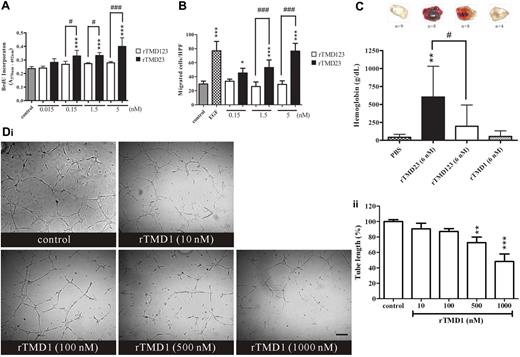
rTMD23 has been shown to induce angiogenesis in vitro and in vivo.7 In this study, the angiogenic properties of rTMD123 and rTMD23 were compared in vitro and in vivo. rTMD23 but not rTMD123 significantly stimulated DNA synthesis and migration of HUVECs (Figure 1A-B). rTMD23 showed higher mitogenic and migratory activity than the same molar concentrations of rTMD123 or the control (Figure 1A-B). These results implied that the lectin-like domain of TM may attenuate the mitogenic and chemotactic activity of rTMD23. By using the Boyden chamber migration assay, we confirmed that migratory activity of rTMD23 diminished in a dose-dependent manner in the presence of rTMD1 (supplemental Figure 2). To compare the angiogenic activity of rTMD23 and rTMD123 in vivo, the angiogenic response to various Matrigel plugs containing rTMD23, rTMD123, rTMD1, or PBS was measured. PBS and rTMD1-containing plugs failed to induce angiogenesis (Figure 1C). The angiogenic response was more prominent to plugs containing rTMD23 than to plugs containing rTMD123 (Figure 1C). In addition, rTMD1 interfered with angiogenesis induced by rTMD23 in the murine Matrigel assay (supplemental Figure 3). These results suggested that rTMD1 may have the potential to inhibit angiogenesis. Therefore, the in vitro tube formation assay was performed to assess this potential. HUVECs formed capillary structures on Matrigel in response to stimulation with mixed factors (serum and endothelial cell growth supplement), and tube formation was decreased by rTMD1 in a concentration-dependent manner (Figure 1D).
The effect of rTMD proteins on angiogenesis in vitro and in vivo. (A) Growth-arrested HUVECs were treated with various Pichia-expressed rTMD proteins for 2 days, and then pulse labeled with BrdU for 2 hours. (B) Chemotaxis was assessed by Boyden chamber migration assay. EGF (10 ng/mL) was used as positive control. HUVECs were added in the upper compartment, and the bottom compartment was filled with various rTMD proteins. After 4 hours of incubation, HUVECs that migrated to lower face of the membrane were counted. HPF indicates high-power field. Values are the mean ± SD (n = 5), and similar results were obtained in at least 3 different experiments. *P < .05 and ***P < .001 compared with the control; #P < .05 and ###P < .001 compared with the rTMD123. (C) rTMD proteins show different angiogenic activity in vivo. Matrigel plugs containing equal molar concentrations of rTMD1, rTMD23, or rTMD123 were used in the murine angiogenesis assay. The amounts of hemoglobin indicated the angiogenic activity elicited by rTMD proteins. The data represent mean ± SD determined from 4-9 plugs. Similar results were obtained from at least 2 separate experiments; n represents the number of plugs for each group. **P < .01 compared with the PBS; #P < .05 compared with the rTMD123. (D) rTMD1 inhibited tube formation on Matrigel. HUVECs in the presence of 5% FBS and 3.75 μg/mL endothelial cell growth supplement were pretreated with various concentrations of rTMD1 for 30 minutes before being added to the Matrigel. After 8 hours of incubation, the tube length was measured using the MetaMorph program. The magnification bar is 100 μm. The data are the mean ± SD (n = 3). **P < .01 and ***P < .001 compared with the control. Similar results were obtained in at least 3 different experiments.
The effect of rTMD proteins on angiogenesis in vitro and in vivo. (A) Growth-arrested HUVECs were treated with various Pichia-expressed rTMD proteins for 2 days, and then pulse labeled with BrdU for 2 hours. (B) Chemotaxis was assessed by Boyden chamber migration assay. EGF (10 ng/mL) was used as positive control. HUVECs were added in the upper compartment, and the bottom compartment was filled with various rTMD proteins. After 4 hours of incubation, HUVECs that migrated to lower face of the membrane were counted. HPF indicates high-power field. Values are the mean ± SD (n = 5), and similar results were obtained in at least 3 different experiments. *P < .05 and ***P < .001 compared with the control; #P < .05 and ###P < .001 compared with the rTMD123. (C) rTMD proteins show different angiogenic activity in vivo. Matrigel plugs containing equal molar concentrations of rTMD1, rTMD23, or rTMD123 were used in the murine angiogenesis assay. The amounts of hemoglobin indicated the angiogenic activity elicited by rTMD proteins. The data represent mean ± SD determined from 4-9 plugs. Similar results were obtained from at least 2 separate experiments; n represents the number of plugs for each group. **P < .01 compared with the PBS; #P < .05 compared with the rTMD123. (D) rTMD1 inhibited tube formation on Matrigel. HUVECs in the presence of 5% FBS and 3.75 μg/mL endothelial cell growth supplement were pretreated with various concentrations of rTMD1 for 30 minutes before being added to the Matrigel. After 8 hours of incubation, the tube length was measured using the MetaMorph program. The magnification bar is 100 μm. The data are the mean ± SD (n = 3). **P < .01 and ***P < .001 compared with the control. Similar results were obtained in at least 3 different experiments.
Interaction of rTMD1 and LeY
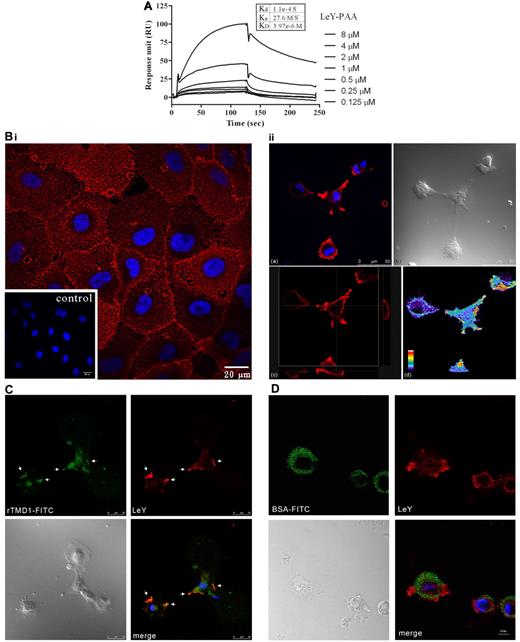
The specific interaction of rTMD1 and LeY has been documented before.11 In this study, the concentration-dependent interaction of LeY and rTMD1 was shown by SPR assay (Figure 2A). In addition, rTMD1Δ but not rTMD23Δ interacted with LeY in the SPR assay (supplemental Figure 4). The equilibrium dissociation constant (KD) estimated from the result of SPR assay was ∼ 3.97 × 10−6 mol/L. The participation of Lewis Ag in endothelial cell-cell contacts has been suggested.18 However, the distribution of LeY on endothelial cells during tubular morphogenesis is unclear. The pattern of LeY expression on the plasma membranes of HUVECs cultured on gelatin-coated surfaces was initially punctuate and evenly distributed (Figure 2B left panel). When HUVECs were cultured on a Matrigel-coated surface within 1 hour of incubation, LeY became concentrated at membrane ruffles and protrusions (Figure 2B right panel and 2C). Monitoring the dynamics of LeY-rTMD1 interaction during the early stage of endothelial tube formation showed that LeY and rTMD1-FITC was concentrated at the membrane ruffles and protrusions (Figure 2C white arrows) and BSA-FITC (Figure 2D) was used as the negative control. The percentage of colocalization estimated from the image was ∼ 89.25% (rTMD1-FITC over LeY) and 36.29% (LeY over rTMD1-FITC) by the MetaMorph program using the integration method. Thus, site-specific localization of LeY on HUVECs may contribute to the formation of endothelial cell-cell contacts and connections.
rTMD1 interacts with LeYAg. (A) SPR measurement. Various concentrations of LeY were passed through a HEK293-expressed rTMD1Δ-conjugated CM5 chip. The x-axis shows flow time, and the y-axis shows interaction strength. (B) Confocal microscopy image shows the distribution of LeY on HUVECs. (Left panel) The expression of LeY while HUVECs were grown on gelatin-coated surface; the inset shows the IgM control. (Right panel) The distribution of LeY on HUVECs during tube formation on Matrigel. Confocal microscopy was used to obtain an XY-Z stack (3-D series). (a) An X-Y frame shows the distribution of LeY on membrane protrusions. (b) A phase-contrast image (bright field) from same area as that in subpanel a. (c) Confocal microscopy image of transverse X-Z and Y-Z sections of HUVECs labeled with LeY Ab on Matrigel, respectively. (d) Confocal microscopy image of a 3-D reconstruction of HUVECs labeled with LeY Ab on Matrigel. Pseudo-color represents the strength of the signal. The strongest signal is displayed in white. (C) Confocal microscopy image shows the interaction of HEK293-expressed rTMD1-FITC and LeY on HUVECs during tube formation on Matrigel. HUVECs were dissociated in buffer from the culture dish by nonenzymatic cell dissociation and incubated with rTMD1-FITC (C) or BSA-FITC (D) on Matrigel for 1 hour. Green, rTMD1-FITC (C) or BSA-FITC (D); red, LeY; blue, nucleus (DAPI stain). Merged channel shows colocalization (white arrows) of rTMD1-FITC and LeY at membrane ruffles and protrusions. A similar result was obtained by assessing 25 cells by confocal microscopy. Representative figures are shown. Observations were repeated in at least 3 separate experiments.
rTMD1 interacts with LeYAg. (A) SPR measurement. Various concentrations of LeY were passed through a HEK293-expressed rTMD1Δ-conjugated CM5 chip. The x-axis shows flow time, and the y-axis shows interaction strength. (B) Confocal microscopy image shows the distribution of LeY on HUVECs. (Left panel) The expression of LeY while HUVECs were grown on gelatin-coated surface; the inset shows the IgM control. (Right panel) The distribution of LeY on HUVECs during tube formation on Matrigel. Confocal microscopy was used to obtain an XY-Z stack (3-D series). (a) An X-Y frame shows the distribution of LeY on membrane protrusions. (b) A phase-contrast image (bright field) from same area as that in subpanel a. (c) Confocal microscopy image of transverse X-Z and Y-Z sections of HUVECs labeled with LeY Ab on Matrigel, respectively. (d) Confocal microscopy image of a 3-D reconstruction of HUVECs labeled with LeY Ab on Matrigel. Pseudo-color represents the strength of the signal. The strongest signal is displayed in white. (C) Confocal microscopy image shows the interaction of HEK293-expressed rTMD1-FITC and LeY on HUVECs during tube formation on Matrigel. HUVECs were dissociated in buffer from the culture dish by nonenzymatic cell dissociation and incubated with rTMD1-FITC (C) or BSA-FITC (D) on Matrigel for 1 hour. Green, rTMD1-FITC (C) or BSA-FITC (D); red, LeY; blue, nucleus (DAPI stain). Merged channel shows colocalization (white arrows) of rTMD1-FITC and LeY at membrane ruffles and protrusions. A similar result was obtained by assessing 25 cells by confocal microscopy. Representative figures are shown. Observations were repeated in at least 3 separate experiments.
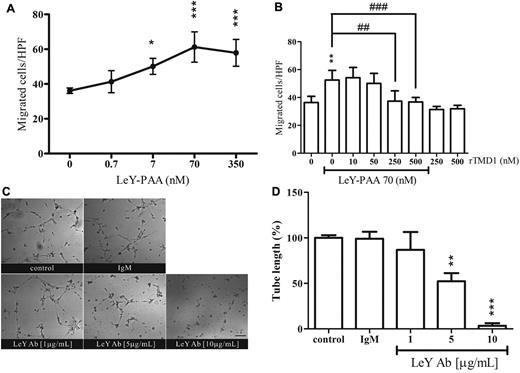
Mediation of the migration and tube formation of HUVECs by LeY and the suppressive effect of rTMD1
Soluble LeY/H has been shown to be chemotactic for human dermal microvascular endothelial cells.15 In this study, LeY-PAA enhanced HUVEC chemotaxis in a concentration-dependent manner (Figure 3A). However, polyacrylamide-based multivalent Lewis X Ag had no chemotactic activity (data not shown). In addition, this activity was diminished in the presence of rTMD1 (Figure 3B). The participation of membrane-bound LeY in endothelial tube formation in retrovirally immortalized human BM endothelial cells has been suggested.18 Therefore, the role of membrane-bound LeY in tube formation was examined in HUVECs. Tube structure was normal in control cultures (ie, in the presence of PBS or isotype-matched Ab) but was concentration-dependently disrupted in the presence of LeY Ab (Figure 3C-D), suggesting that LeY participates in angiogenic processes, and the interaction of rTMD1 with concentrated LeY on the membrane ruffles (Figure 2C) may contribute to the inhibitory effect of rTMD1 on vascular tube formation in vitro (Figure 1D).
LeY has angiogenic properties and its effect is attenuated by rTMD1. (A-B) Chemotactic effect of LeY-PAA. Boyden chamber migration assay of HUVECs was performed. (A) Various concentrations of LeY-PAA were added to the bottom wells as chemoattractant, and migrated HUVECs were enumerated after 4 hours of incubation. Values are the mean ± SD (n = 5). *P < .05 and ***P < .001 compared with the vehicle control. (B) LeY-PAA was incubated with various concentrations of Pichia-expressed rTMD1 30 minutes before being added to bottom wells as chemoattractant. Values are the mean ± SD (n = 5), and similar results were obtained in at least 3 different experiments. *P < .05, **P < .01, and ***P < .001 compared with the control. ##P < .01 and ###P < .001 compared with the LeY-PAA. HPF indicates high-power field. (C-D) The effect of LeY Ab on tube formation. HUVECs were preincubated with various concentrations of LeY Ab or isotype-matched IgM 30 minutes before being placed on Matrigel. (C) Schematic presentation of tube structure on Matrigel after 6 hours of incubation. The magnification bar is 100 μm. (D) Statistical analysis of the total tube length. Mouse IgM (10 μg/mL) was used as isotype-matched control. The data are the mean ± SD (n = 3). **P < .01 and ***P < .001 compared with the IgM. Similar results were obtained in at least 3 different experiments.
LeY has angiogenic properties and its effect is attenuated by rTMD1. (A-B) Chemotactic effect of LeY-PAA. Boyden chamber migration assay of HUVECs was performed. (A) Various concentrations of LeY-PAA were added to the bottom wells as chemoattractant, and migrated HUVECs were enumerated after 4 hours of incubation. Values are the mean ± SD (n = 5). *P < .05 and ***P < .001 compared with the vehicle control. (B) LeY-PAA was incubated with various concentrations of Pichia-expressed rTMD1 30 minutes before being added to bottom wells as chemoattractant. Values are the mean ± SD (n = 5), and similar results were obtained in at least 3 different experiments. *P < .05, **P < .01, and ***P < .001 compared with the control. ##P < .01 and ###P < .001 compared with the LeY-PAA. HPF indicates high-power field. (C-D) The effect of LeY Ab on tube formation. HUVECs were preincubated with various concentrations of LeY Ab or isotype-matched IgM 30 minutes before being placed on Matrigel. (C) Schematic presentation of tube structure on Matrigel after 6 hours of incubation. The magnification bar is 100 μm. (D) Statistical analysis of the total tube length. Mouse IgM (10 μg/mL) was used as isotype-matched control. The data are the mean ± SD (n = 3). **P < .01 and ***P < .001 compared with the IgM. Similar results were obtained in at least 3 different experiments.
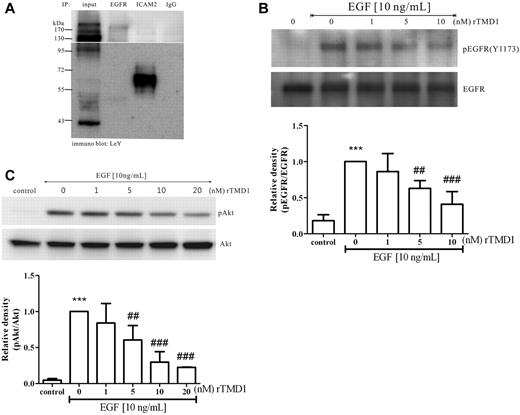
Inhibitory effect of rTMD1 on angiogenic signaling elicited by EGF
Modification of EGFR by LeY in several human carcinomas,13 interference with EGFR signaling by Ab against LeY,25 and promotion of angiogenesis by EGFR ligands have been demonstrated previously.26 Because LeY was highly expressed on the cell surface of HUVECs (Figure 2) and had contributed to migration and tube formation in HUVECs in vitro (Figure 3), we examined whether LeY modified EGFR on HUVECs. Immunoprecipitation assays showed that several glycoproteins including EGFR and ICAM2 carried LeY (Figure 4A). We further tested whether rTMD1 would interfere with EGF-induced angiogenesis. The EGF-induced signaling pathways leading to angiogenesis involve EGFR phosphorylation and the activation of various downstream effectors, such as ERK and Akt.27 When HUVECs were pretreated with rTMD1, EGF-induced EGFR phosphorylation and the activation of Akt were inhibited in a concentration-dependent manner (Figure 4B-C, supplemental Figures 5-6).
rTMD1 inhibits EGF-mediated signaling in HUVECs. (A) EGFR was modified by LeY. HUVECs lysate was immunoprecipitated with Abs against EGFR, ICAM2, or IgG, and then immunoblotted with LeY Ab. ICAM2 acts as a positive control and IgG as a negative control. (B-C) HUVECs were preincubated with various concentrations of Pichia-expressed rTMD1 for 30 minutes, and then stimulated with 10 ng/mL EGF for 10 minutes. (B) rTMD1 concentration-dependently inhibited phosphorylation of tyrosine 1173 on EGFR. EGFR in HUVECs lysate was immunoprecipitated with EGFR Ab, and then immunoblotted with an Ab against the phosphor-tyrosine1173 on EGFR. (C) rTMD1 decreased activation of Akt induced by EGF. The similar results were obtained in at least 3 different experiments, and the relative density of each band was quantified and normalized. The densitometry values are mean ± SD. ***P < .001 compared with the control; ##P < .01 and ###P < .001 compared with EGF alone.
rTMD1 inhibits EGF-mediated signaling in HUVECs. (A) EGFR was modified by LeY. HUVECs lysate was immunoprecipitated with Abs against EGFR, ICAM2, or IgG, and then immunoblotted with LeY Ab. ICAM2 acts as a positive control and IgG as a negative control. (B-C) HUVECs were preincubated with various concentrations of Pichia-expressed rTMD1 for 30 minutes, and then stimulated with 10 ng/mL EGF for 10 minutes. (B) rTMD1 concentration-dependently inhibited phosphorylation of tyrosine 1173 on EGFR. EGFR in HUVECs lysate was immunoprecipitated with EGFR Ab, and then immunoblotted with an Ab against the phosphor-tyrosine1173 on EGFR. (C) rTMD1 decreased activation of Akt induced by EGF. The similar results were obtained in at least 3 different experiments, and the relative density of each band was quantified and normalized. The densitometry values are mean ± SD. ***P < .001 compared with the control; ##P < .01 and ###P < .001 compared with EGF alone.
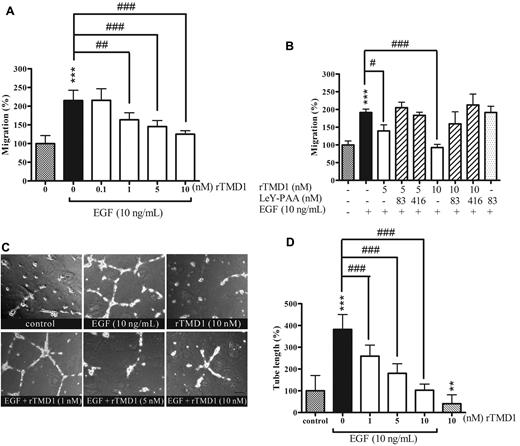
Inhibitory effect of rTMD1 on EGF-mediated HUVEC migration and tube formation in vitro
Directional migration of endothelial cells toward angiogenic factors is crucial to angiogenesis. EGF elicits cell activation that leads to cell migration.27 To explore whether the inhibition of EGF-induced signaling by rTMD1 could affect cell migration and tube formation, the effects of rTMD1 on EGF-mediated angiogenesis in vitro and in vivo were investigated. The chemotaxis of HUVECs toward EGF was significantly inhibited by rTMD1 in a concentration-dependent manner (Figure 5A). The inhibition by rTMD1 on HUVEC migration toward EGF was abolished when rTMD1 was preincubated with multivalent LeY (Figure 5B), suggesting that the interaction of rTMD1 and LeY is responsible for the inhibitory effect of rTMD1. Moreover, rTMD1 not only suppressed the basal level of tube formation, but also inhibited EGF-elicited tube formation on Matrigel in a concentration-dependent manner (Figure 5C-D). Furthermore, the inhibitory effect of rTMD1Δ was similar to that of rTMD1 on HUVEC chemotaxis (supplemental Figure 7) induced by EGF.
rTMD1 suppresses EGF-mediated HUVEC migration and tube formation. (A) Pichia-expressed rTMD1 inhibits EGF-mediated HUVEC migration. EGF-containing media was added to the bottom compartment of a Boyden chamber as chemoattractant, and rTMD1 was preincubated with HUVECs 30 minutes before being added to the upper compartment. (B) Multivalent LeY Ag blocks the rTMD1-mediated inhibitory effect on EGF-elicited endothelial cell migration. The Boyden chamber assay was performed. rTMD1 was mixed with multivalent LeY (LeY-PAA) in cell solution, and EGF was used as chemoattractant. ***P < .001 compared with the control. #P < .05, ##P < .01, and ###P < .001 compared with EGF alone. The data are the mean ± SD (n = 6). Similar results were obtained in at least 3 independent experiments. (C-D) rTMD1 inhibits tube formation induced by EGF. (C) Schematic presentation of tube structure on Matrigel after 14 hours of incubation. (D) Statistical analysis of the tube length in each group. The magnification bar is 20 μm. The data are the mean ± SD (n = 3). Similar results were obtained in at least 3 independent experiments. **P < .01 and ***P < .001 compared with the control; ###P < .001 compared with EGF alone.
rTMD1 suppresses EGF-mediated HUVEC migration and tube formation. (A) Pichia-expressed rTMD1 inhibits EGF-mediated HUVEC migration. EGF-containing media was added to the bottom compartment of a Boyden chamber as chemoattractant, and rTMD1 was preincubated with HUVECs 30 minutes before being added to the upper compartment. (B) Multivalent LeY Ag blocks the rTMD1-mediated inhibitory effect on EGF-elicited endothelial cell migration. The Boyden chamber assay was performed. rTMD1 was mixed with multivalent LeY (LeY-PAA) in cell solution, and EGF was used as chemoattractant. ***P < .001 compared with the control. #P < .05, ##P < .01, and ###P < .001 compared with EGF alone. The data are the mean ± SD (n = 6). Similar results were obtained in at least 3 independent experiments. (C-D) rTMD1 inhibits tube formation induced by EGF. (C) Schematic presentation of tube structure on Matrigel after 14 hours of incubation. (D) Statistical analysis of the tube length in each group. The magnification bar is 20 μm. The data are the mean ± SD (n = 3). Similar results were obtained in at least 3 independent experiments. **P < .01 and ***P < .001 compared with the control; ###P < .001 compared with EGF alone.
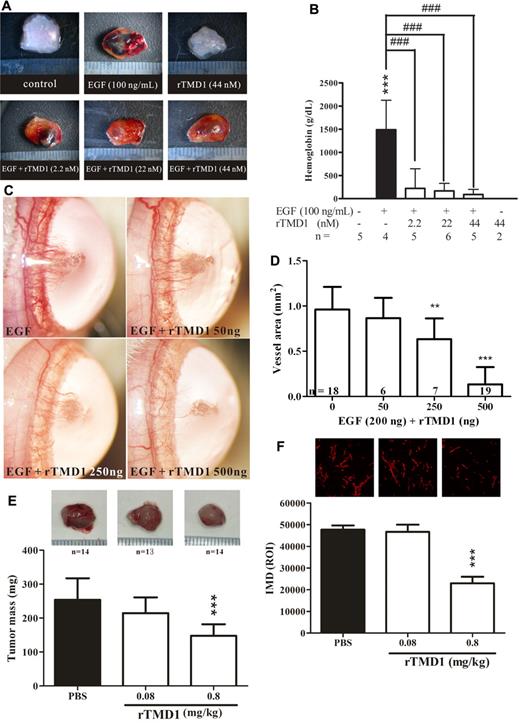
Inhibitory effect of rTMD1 on angiogenesis in vivo
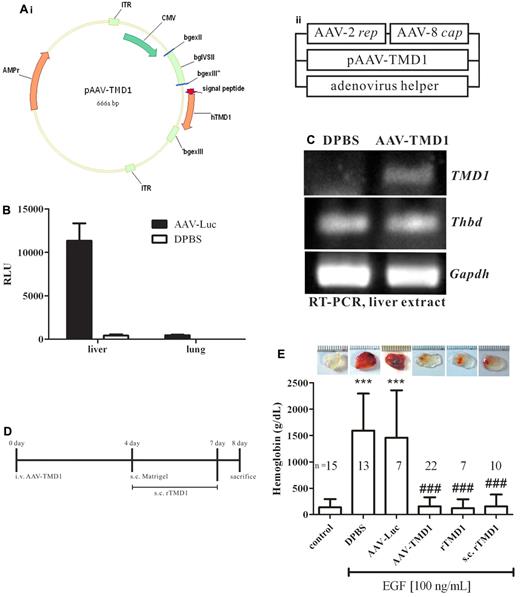
Because rTMD1 exhibited antiangiogenic activity in vitro, its corresponding activity in vivo was examined by using the murine Matrigel implantation assay, a rat corneal micropocket assay, and the tumor angiogenesis assay. Control Matrigel plugs and those containing rTMD1 were pale in color and contained little hemoglobin, whereas those with EGF contained areas of prominent angiogenesis (Figure 6A-B), which were decreased in the presence of rTMD1 (Figure 6A-B). A similar result was obtained by using rTMD1Δ (supplemental Figure 8). In the rat corneal micropocket assay, neovascularization in the cornea was significantly induced 7 days after implantation of a pellet containing 200 ng of EGF, whereas neovascularization was dose-dependently reduced in the presence of rTMD1 (Figure 6C-D). Tumor growth is enhanced by angiogenesis, and tumor angiogenesis contributes to the conversion of micrometastasis to deadly macrometastasis.28 Several angiogenic factors are involved in tumor angiogenesis, including vascular endothelial growth factor, basic fibroblast growth factor, and EGF.28 rTMD1 effectively inhibited EGF-mediated angiogenesis in vitro and in vivo (Figures 1D,4B–6D). Furthermore, we tested the effect of rTMD1 on tumor angiogenesis by using xenografts of Lewis lung carcinoma cells in mice. Tumor growth was reduced in animals treated with 0.8 mg/kg rTMD1 (Figure 6E) daily for 2 weeks. Histologic examination by CD31 staining showed reduced intratumoral microvessel density in the group treated with 0.8 mg/kg rTMD1 (Figure 6F), suggesting that the antiangiogenic activity of rTMD1 may contribute to its tumor suppressive effect. In addition, the efficacy of different methods of administration of rTMD1 on EGF-mediated angiogenesis in vivo was evaluated by Matrigel assay (Figure 7). Recombinant AAV vectors are a promising alternative for long-term tissue-specific protein expression.29 The AAV serotype 2/8 expression vector was used to make mouse hepatocytes secrete TMD1 constitutively. The tissue-specific infection with AAV serotype 2/8 was examined by the luciferase assay. We confirmed that luciferase activity was detected in the liver extract but not in the lung extract of mice infected with AAV-Luc (Figure 7B). The expression of AAV-TMD1 was examined by RT-PCR with primers specific for the human lectin-like domain. TMD1 was specifically expressed in the liver of mice infected with AAV-TMD1 (Figure 7C). A similar antiangiogenesis effect was observed with a subcutaneous injection of rTMD1 around the Matrigel once daily for 4 days, an IV infusion of AAV-TMD1 4 days before Matrigel implantation, or a coinjection of rTMD1 and EGF in the Matrigel (Figure 7D-E).
rTMD1 inhibits angiogenesis in vivo. (A-B) Pichia-expressed rTMD1 inhibits angiogenesis in vivo using a murine Matrigel assay. (A) Schematic representation of Matrigel plugs. The magnification bar is 1 mm. (B) Statistical analysis of murine angiogenic assay. The data represent mean ± SD. Similar results were obtained in 3 independent experiments; n represents the number of plugs for each group. ***P < .001 compared with the PBS. ###P < .001 compared with EGF alone. (C-D) rTMD1 inhibits angiogenesis in vivo in a rat corneal micropocket assay. A micropocket in the cornea was surgically generated and implanted with a pellet containing various combinations of 200 ng of EGF and various amounts of Pichia-expressed rTMD1. (C) Corneal photographs were taken 7 days after implantation. Representative figures are shown. (D) Statistical analysis of corneal neovascularization. The data represent mean ± SD. Similar results were obtained in 3 independent experiments; n represents the number of eyes in each group. **P < .01 and ***P < .001 compared with EGF alone. (E-F) rTMD1 inhibits tumor angiogenesis. (E) Lewis lung carcinoma cells (1 × 106) were administered to C57BL/6 mice by subcutaneous dorsal injection. The mice were given daily IP injections of various concentrations of Pichia-expressed rTMD1 for 2 weeks. Representative figures and statistical analyses of tumor mass are shown. ***P < .001 compared with the PBS control. The data represent mean ± SD; n represents the number of mice in each group. (F) Analysis of intratumoral microvessel density (IMD). IMD was detected by CD31 staining. The density of microvessels was quantified in the most vascular areas of the tumor. ***P < .001 compared with the PBS group. The data represent mean ± SD.
rTMD1 inhibits angiogenesis in vivo. (A-B) Pichia-expressed rTMD1 inhibits angiogenesis in vivo using a murine Matrigel assay. (A) Schematic representation of Matrigel plugs. The magnification bar is 1 mm. (B) Statistical analysis of murine angiogenic assay. The data represent mean ± SD. Similar results were obtained in 3 independent experiments; n represents the number of plugs for each group. ***P < .001 compared with the PBS. ###P < .001 compared with EGF alone. (C-D) rTMD1 inhibits angiogenesis in vivo in a rat corneal micropocket assay. A micropocket in the cornea was surgically generated and implanted with a pellet containing various combinations of 200 ng of EGF and various amounts of Pichia-expressed rTMD1. (C) Corneal photographs were taken 7 days after implantation. Representative figures are shown. (D) Statistical analysis of corneal neovascularization. The data represent mean ± SD. Similar results were obtained in 3 independent experiments; n represents the number of eyes in each group. **P < .01 and ***P < .001 compared with EGF alone. (E-F) rTMD1 inhibits tumor angiogenesis. (E) Lewis lung carcinoma cells (1 × 106) were administered to C57BL/6 mice by subcutaneous dorsal injection. The mice were given daily IP injections of various concentrations of Pichia-expressed rTMD1 for 2 weeks. Representative figures and statistical analyses of tumor mass are shown. ***P < .001 compared with the PBS control. The data represent mean ± SD; n represents the number of mice in each group. (F) Analysis of intratumoral microvessel density (IMD). IMD was detected by CD31 staining. The density of microvessels was quantified in the most vascular areas of the tumor. ***P < .001 compared with the PBS group. The data represent mean ± SD.
AAV-TMD1 suppresses experimental angiogenesis in vivo. (A) Schematic representation of the construction of pAAV-TMD1 and generation of AAV-TMD1 particles by tripartite transfection of 3 plasmids into 293A cells. (B) Luciferase assay. Equivalent samples were analyzed after inoculation of the mice with AAV-Luc or DPBS. RLU indicates relative light unit. (C) RT-PCR of liver extract after inoculation of mice with AAV-TMD1 or DPBS. TMD1: human TMD1, 381 bp; Thbd: mouse TM, 130 bp; Gapdh: mouse GAPDH, 601 bp. (D) Experimental protocol. FVB mice were divided into 6 groups; 1 was given a single injection of AAV-TMD1, DPBS, or AAV-Luc 4 days before implantation of Matrigel; was given a subcutaneous injection of 200 μL of Pichia-expressed rTMD1 (80 μg/mL) daily from day 4 to day 7, and the others were given Matrigel containing EGF with or without rTMD1 (44 nmol/L). On day 8, the mice were killed and the lung, liver, and Matrigel were collected. The angiogenic index was evaluated by the hemoglobin content in the Matrigel. (E) Statistical analysis of the murine angiogenic assay. The data represent mean ± SD. Similar results were obtained in 3 independent experiments; n represents the number of plugs for each group. ***P < .001 compared with the control. ###P < .001 compared with the DPBS or AAV-Luc. s.c. indicates subcutaneously; and i.v., intravenously.
AAV-TMD1 suppresses experimental angiogenesis in vivo. (A) Schematic representation of the construction of pAAV-TMD1 and generation of AAV-TMD1 particles by tripartite transfection of 3 plasmids into 293A cells. (B) Luciferase assay. Equivalent samples were analyzed after inoculation of the mice with AAV-Luc or DPBS. RLU indicates relative light unit. (C) RT-PCR of liver extract after inoculation of mice with AAV-TMD1 or DPBS. TMD1: human TMD1, 381 bp; Thbd: mouse TM, 130 bp; Gapdh: mouse GAPDH, 601 bp. (D) Experimental protocol. FVB mice were divided into 6 groups; 1 was given a single injection of AAV-TMD1, DPBS, or AAV-Luc 4 days before implantation of Matrigel; was given a subcutaneous injection of 200 μL of Pichia-expressed rTMD1 (80 μg/mL) daily from day 4 to day 7, and the others were given Matrigel containing EGF with or without rTMD1 (44 nmol/L). On day 8, the mice were killed and the lung, liver, and Matrigel were collected. The angiogenic index was evaluated by the hemoglobin content in the Matrigel. (E) Statistical analysis of the murine angiogenic assay. The data represent mean ± SD. Similar results were obtained in 3 independent experiments; n represents the number of plugs for each group. ***P < .001 compared with the control. ###P < .001 compared with the DPBS or AAV-Luc. s.c. indicates subcutaneously; and i.v., intravenously.
Discussion
Many angiogenesis-related factors are proteolytically cleaved from their preexisting precursors. For example, the proangiogenic molecule EGF is generated and released from larger membrane-anchored precursors by proteolytic processing.30 The antiangiogenic molecule angiostatin is generated from plasminogen after sequential cleavage of peptide bonds by several proteases.31 Moreover, 2 proteolytic fragments exerting opposite effects on angiogenesis are derived from thrombospondin-1 (TSP-1). A 25-kDa fragment of TSP-1 (like the whole TSP-1) promotes angiogenesis. In contrast, a 140-kDa fragment of TSP-1 inhibits angiogenesis elicited by basic fibroblast growth factor.32 In both this study and a previous report, we demonstrated that, like TSP-1, TM possesses both angiogenic and antiangiogenic activities. rTMD23 exhibited mitogenic and angiogenic activity.7 In contrast, rTMD1 showed antiangiogenic activity through the interaction with LeY. The results showed that LeY is highly expressed on the cell surface of HUVECs and is concentrated in the membrane ruffles and protrusions under conditions of tubular morphogenesis on Matrigel. The interaction of rTMD1 with LeY may lead to the inhibition of HUVEC chemotaxis (Figures 2–3) and to the suppression of tube formation on Matrigel (Figure 1D). In addition, rTMD1 interfered with LeY-modified EGFR, resulting in suppression of EGF-mediated angiogenesis in vitro and in vivo. The results implied that LeY may participate in the connection and formation of capillaries and that rTMD1 interferes with angiogenesis.
The level of plasma TM has been used as an indicator of endothelial damage in myocardial infarction,33 sepsis,34 and acute coronary syndrome.35 Studies of recombinant TM proteins or native soluble TM fragments from body fluid suggested that soluble TM not only serves as a marker for endothelial damage, but also has some physiologic or pathophysiologic effects. The recombinant EGF-like domain of TM (TM domain 2, rTMD2) has a mitogenic effect on several cell types, including smooth muscle cells and Swiss 3T3 cells.36,37 Furthermore, differences in angiogenic activity between rTMD proteins (rTMD123, rTMD23, rTMD2, and rTMD1) were shown in a previous study and this report.7 rTMD123 seems to have some angiogenic activity in the murine angiogenesis assay, although it was not statistically significant versus the control (Figure 1C). The small difference between the result of the in vitro cell proliferation and migration assays and those of the in vivo angiogenic assay of rTMD123 (Figure 1A-C) may be because of limitations of experimental sensitivity, dosage of recombinant protein, and the experimental time window, and implies that rTMD123 in solution may assume a tertiary structure that diminishes the angiogenic activity of TM. These observations are consistent with our finding that rTMD1 inhibited rTMD23-mediated chemotaxis of HUVECs (supplemental Figure 2) and angiogenesis in vivo (supplemental Figure 3). The mechanism underlying the inhibitory effect of rTMD1 on rTMD23-induced angiogenesis may be mediated through interaction with LeY because we observed strong induction and localization of LeY on the membrane ruffles and protrusions of rTMD23-stimulated HUVECs on Matrigel (data not shown). rTMD1 suppresses inflammation by interfering with the NF-κB and MAPK pathway,8 sequestering high-mobility group-B1 protein,9 inhibiting complement activation,10 and interaction with LeY.11 In general, the lectin-like domain of TM inhibits inflammation, and the EGF-like domain of TM promotes cell growth and migration. However, little is known about the kinds of soluble TM fragments generated and how they are generated. Generation of Abs against different TM domains may be useful for detecting and distinguishing the molecular species of soluble TM fragments. However, these Abs are not commercially available to date.
The role of LeY in endothelial tube formation and angiogenesis has been demonstrated previously.15,18 Our results support the role of LeY in angiogenesis and provide direct evidence of the distribution of LeY on endothelial cells during vascular tube formation (Figure 2). LeY in endothelial cells is rapidly mobilized from the cytoplasm to plasma membrane on inflammatory stimulation, and soluble LeY is increased in synovial fluid from patients with rheumatoid arthritis.15 In addition, treatment of mice with rTMD1 protects against arthritis through interfering with complement activation.10 In this study, we found a role for LeY in tube formation that suggests another possible mechanism of rTMD1-mediated protection against arthritis (ie, through sequestration of soluble LeY by rTMD1). The importance of LeY in tube formation was supported by our demonstration that Ab against LeY can disrupt tube formation (Figure 3). In addition, the interaction of rTMD1 with membrane-bound LeY on membrane ruffles and protrusions may be responsible for the inhibitory effect of rTMD1 on tube formation in vitro (Figures 1D,2C).
Many kinds of mammalian and plant lectins interfered with EGFR signaling by reducing ligand binding and receptor activation.38,39 Moreover, LeY modification of the EGFR family of receptor tyrosine kinases has been suggested to mediate signal transduction and tumor progression.25,40 rTMD1 has a C-type lectin-like motif that may interact with mannose, chondroitin sulfate A/C, and LeY Ag.11,19 Herein, we demonstrated that rTMD1 may interact with LeY-modified EGFR and thereby diminish EGF-mediated EGFR signaling and angiogenesis.
The wider expression of TM extravascularly41 and the critical effect of TM on embryogenesis suggest that TM may have functions besides anticoagulation.42 Clinical studies have tried to link lower TM expression in cancer tissue to worse prognosis and a high incidence of metastasis.43,44 Recently, the TM-regulated hemostatic system was demonstrated to be important for the survival of tumor cells newly localized to the lung.45 Our previous study also demonstrated that overexpression of TM but not lectin-like domain-deleted TM in TM-negative melanoma cells reduced tumor growth in vivo.19 This result is consistent with the present study that the lectin-like domain of TM inhibits angiogenesis, thereby reducing tumor growth. In addition, native TM fragments from human urine containing lectin-like structure had an antimetastatic effect in a murine experimental metastasis model, suggesting the role of lectin-like domain of TM on antimetastasis.46 These studies imply that TM may be a tumor suppressor that exerts its effect via regulation of thrombin through its EGF-like domain and the suppression of inflammation and angiogenesis through its lectin-like domain. The later hypothesis was supported by our finding that rTMD1 could suppress tumor growth in vivo by inhibiting tumor angiogenesis (Figure 6E-F). However, the exact mechanism underlying lower expression of TM on tumor tissues is unclear. Matrix metalloproteinases have been suggested to be involved in lysophosphatidic acid–mediated shedding of the TM lectin-like domain.47 Presumably, soluble TM fragments are generated through proteolytic degradation, which may occur during inflammation and in the tumor microenvironment.
In conclusion, our evidence supports the role of LeY in angiogenesis, where LeY contributes to EGFR signaling, endothelial cell-cell contacts, chemotaxis, and tube formation. rTMD1 inhibits angiogenesis through interaction with and sequestration of LeY. Because the recombinant AAV system showed promise for the efficient, long-term in vivo expression of genes of interest and showed no pathogenic effect on the host, the use of AAV-TMD1 holds promise for the treatment of diseases associated with chronic inflammation and angiogenesis. We generated AAV-TMD1 and successfully demonstrated its effect on antiangiogenesis in vivo as rTMD1, providing the preclinical information required for use in humans and in further investigations. In addition, these results highlight a novel function of TM domains in the modulation of angiogenesis and also illuminate a possible route for antiangiogenic therapy.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Science Council (NSC), Executive Yuan, Taipei, Taiwan, grants NSC 97-2752-B-006-003-PAE, NSC 99-2320-B-006-008-MY3, and NSC 99-2628-B-006-003-MY3.
Authorship
Contribution: C.-H.K. designed and performed the research and wrote the manuscript; P.-K.C., B.-I.C., M.-C.S., and C.-S.S. contributed vital reagents and analytical tools; P.-K.C. and C.-F.C. performed the SPR assay; J.-S.L. designed and produced AAV expression system; and G.-Y.S. and H.-L.W. designed and wrote the manuscript, and supervised the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for J.-S.L. is AB Biosciences Inc, Allston, MA.
Correspondence: Hua-Lin Wu, Department of Biochemistry and Molecular Biology, College of Medicine, National Cheng Kung University, 1 University Rd, Tainan 701, Taiwan; e-mail: halnwu@mail.ncku.edu.tw; or Guey-Yueh Shi, Department of Biochemistry and Molecular Biology, College of Medicine, National Cheng Kung University, 1 University Rd, Tainan 701, Taiwan; e-mail: gyshi@mail.ncku.edu.tw.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal