Abstract
The genetic engineering of hematopoietic stem cells is the basis for potentially treating a large array of hereditary and acquired diseases, and stands as the paradigm for stem cell engineering in general. Recent clinical reports support the formidable promise of this approach but also highlight the limitations of the technologies used to date, which have on occasion resulted in clonal expansion, myelodysplasia, or leukemogenesis. New research directions, predicated on improved vector designs, targeted gene delivery or the therapeutic use of pluripotent stem cells, herald the advent of safer and more effective hematopoietic stem cell therapies that may transform medical practice. In this review, we place these recent advances in perspective, emphasizing the solutions emerging from a wave of new technologies and highlighting the challenges that lie ahead.
Introduction
The safe engineering and engraftment of hematopoietic stem cells (HSCs) are the keys to treating a vast spectrum of genetic and acquired disorders that affect hematopoietic and other tissues. These include disorders of the immune system, such as severe combined immunodeficiency (SCID) syndromes and AIDS, the thalassemias, sickle cell anemia, metabolic disorders, including central nervous system pathologies, autoimmune diseases, and an array of hematologic malignancies, which could be treated with cancer-free autologous cells or prevented (eg, in the case of Fanconi anemia).1,2 The safe and effective engineering of HSCs thus represents one of the central goals of stem cell and gene therapies. Since the pioneering studies in adenosine deaminase (ADA) deficiency initiated at the National Institutes of Health in the early 1990s, nearly 100 patients have been treated with genetically modified CD34+ hematopoietic progenitors worldwide (Table 1). This slow adaptation reflects both the complexity of the biologic challenges posed by the ex vivo manipulation and genetic engineering of HSCs as well as the chilling impact of the first report of a leukemic transformation caused by a γ-retroviral vector in a boy with X-linked SCID (X-SCID).3 The series of leukemias that ensued (in 5 of 20 patients with X-SCID), which were later followed by similar adverse events in trials for chronic granulomatous disease and Wiscott-Aldrich syndrome, raised serious doubts as to the merits of this approach and undermined human and financial investments in this field over the past decade. However, these serious adverse events also spurred an enormous, collective investigation into the genotoxicity of gene delivery methodologies, resulting in tremendous progress in our understanding of retroviral vector integration and its impact on endogenous gene structure and function.4-6 Although these serious adverse events have resulted in discontinuation of the use of long terminal repeat (LTR)–driven γ-retroviral vectors for the genetic modification of HSCs, they provided a major impetus for developing novel approaches to genetically modify human cells.
Patients treated with engineered HSCs
| Disease . | Vector type . | LTR-driven . | No. of treated patients . |
|---|---|---|---|
| ADA | γRV | + | 40 |
| Gaucher | αRV | + | 3 |
| X-SCID | γRV/SIN-γRV | +/− | 20/3 |
| CGD | γRV | + | 6 |
| ALD | SIN-LV | + | 4 |
| WAS | γRV/SIN-LV | +/− | 10/3 |
| β-thal | SIN-LV | − | 2 |
| MLD | SIN-LV | − | 4 |
| Disease . | Vector type . | LTR-driven . | No. of treated patients . |
|---|---|---|---|
| ADA | γRV | + | 40 |
| Gaucher | αRV | + | 3 |
| X-SCID | γRV/SIN-γRV | +/− | 20/3 |
| CGD | γRV | + | 6 |
| ALD | SIN-LV | + | 4 |
| WAS | γRV/SIN-LV | +/− | 10/3 |
| β-thal | SIN-LV | − | 2 |
| MLD | SIN-LV | − | 4 |
These new trends in HSC engineering broadly fall into 3 categories: improvements in the design of retroviral vectors, development of technologies for targeted gene delivery, and novel approaches made possible by the advent of patient-specific pluripotent stem cells. Some of these have recently entered the clinical arena and others are soon to follow. This review briefly summarizes the first 2 decades of HSC gene therapy, based on the use of first-generation (ie, LTR-driven) γ-retroviral vectors, and critically assesses future directions from the perspective of genetic engineering, examining the prospects, and challenges that lie ahead.
LTR-driven vectors
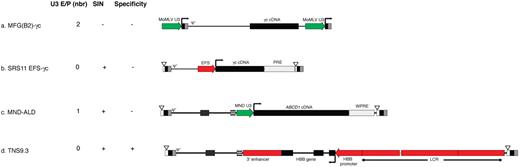
Successes in HSC gene therapy have slowly but steadily accumulated over the past decade. Most early trials focused on severe monogenic immune deficiencies, including ADA deficiency, X-SCID, chronic granulomatous deficiency (CGD), and, more recently, Wiskott-Aldrich syndrome (WAS). These severe disorders were chosen in part because ubiquitous expression of the therapeutic protein (the ADA enzyme for ADA deficiency, the interleukin receptor common γ-chain for X-SCID, the gp91phox oxidase complex protein for CGD, and the WAS signaling integrator protein for WAS), in all hematopoietic cells, not just the defective cell types, was deemed to be acceptable, thus justifying the use of the “first-generation” vectors available in the early 1990s. These consist of recombinant replication-incompetent γ-retroviral genomes derived from murine leukemia viruses (MLVs), termed LXSN,7 MFG/SFG,8 and FMEV,9 which provide constitutive expression of the therapeutic cDNA driven by the viral enhancer/promoter present in the vector's 5′- and 3′-LTRs (Figure 1).
Retroviral vector designs under clinical evaluation. (A) LTR-driven γ-RV, exemplified by the MFG/SFG vector design used in X-SCID and WAS clinical trials. (B) SIN-γ-RV, exemplified by the SRS11 EFS vector design used in the X-SCID consortium trial. (C) Nonspecific SIN-LV, exemplified by the MND-ALD vector design used in the ALD trial. (D) Lineage-restricted SIN-LV, exemplified by the TNS9.3 vector for the treatment of β-thalassemia major. U3 E/P indicates retroviral enhancer/promoter from the LTR U3 region; PRE/WPRE (woodchuck hepatitis), posttranscriptional regulatory element; SIN, self-inactivating vector design (▿ represents U3 deletion); specificity: − indicates ubiquitous; and +, lineage-specific; LCR, locus control region; and HBB, human β-globin gene. Green represents retroviral enhancer/promoter elements; and red, mammalian enhancer/promoter elements.
Retroviral vector designs under clinical evaluation. (A) LTR-driven γ-RV, exemplified by the MFG/SFG vector design used in X-SCID and WAS clinical trials. (B) SIN-γ-RV, exemplified by the SRS11 EFS vector design used in the X-SCID consortium trial. (C) Nonspecific SIN-LV, exemplified by the MND-ALD vector design used in the ALD trial. (D) Lineage-restricted SIN-LV, exemplified by the TNS9.3 vector for the treatment of β-thalassemia major. U3 E/P indicates retroviral enhancer/promoter from the LTR U3 region; PRE/WPRE (woodchuck hepatitis), posttranscriptional regulatory element; SIN, self-inactivating vector design (▿ represents U3 deletion); specificity: − indicates ubiquitous; and +, lineage-specific; LCR, locus control region; and HBB, human β-globin gene. Green represents retroviral enhancer/promoter elements; and red, mammalian enhancer/promoter elements.
In terms of therapeutic efficacy, the overall clinical results have been compelling. The majority of patients with SCID, ADA, and WAS showed dramatic improvements in their immune function, including improved T- and B-cell immunity, as well as restored natural killer cell function in X-SCID and regression of eczema and thrombocytopenia in WAS.10-14 Quality of life was improved for the majority of these patients.15 The outcome in CGD, a myeloid disorder in contrast to the aforementioned lymphoid syndromes, and where adults rather than children were treated, was less dramatic but still resulted in short-term regression or stabilization of intractable infections in the first 2 treated subjects.16
Using LTR-driven expression in another class of retroviral vectors derived from HIV-1, promising clinical results were recently obtained in 2 children with adrenoleukodystrophy (ALD), a disease characterized by multifocal brain demyelination. The 2 first patients showed neuroradiologic improvement and stabilization of their declining cognitive functions.17 Although the observation period is still limited (∼ 3 years), these results bode very well for this class of vectors, which are currently entering the clinic for WAS and other metabolic disorders, including metachromatic leukodystrophy, and β-thalassemia (Table 1). Like the first-generation γ-retroviral vectors, the lentiviral vectors used in the ALD study express the protein nonspecifically in all hematopoietic lineages, including the myeloid cells that eventually reconstitute the brain microglia.18 Significantly, these vectors lack the duplicated full-length LTR that is characteristic of the early γ-RVs, although they still encode an LTR as their internal promoter (Figure 1).
Altogether, these studies support the feasibility of genetically engineering HSCs for use in an autologous setting and the notion that genetically engineered HSCs could provide substantial benefits to patients with a broad range of inherited and acquired disorders.
Current technologies show their limits
Just as the benefits of retroviral therapies were starting to be revealed, so were the shortcomings of gene transfer into stem cells. These include limitations of therapeutic efficacy and toxicities, especially genotoxicity. Some of these problems are inherent to stem cell harvest and cell culture, whereas others are disease- or vector-specific. A successful therapy requires polyclonal hematopoietic reconstitution by self-renewing HSCs, with a sufficient fraction of engrafting HSCs that harbor the vector and expression of the corrective genetic material on a per-cell basis that reaches over the thresholds required to therapeutically impact on the underlying disease over the long term.
Several obstacles of a quantitative nature can interfere with this objective. Effective retroviral transduction of HSCs requires having enough patient CD34+ cells and adequate vector stocks for their transduction. Although CD34+ cell collection from bone marrow or mobilized blood is usually satisfactory, it may be challenging in some conditions, such as Fanconi anemia19 and sickle cell anemia.20
Current approaches to vector transduction require ex vivo culture of CD34+ cells in the presence of pro-survival cytokines, and even short-term culture results in decreased engraftment and ability to compete with endogenous HSCs, perhaps resulting from loss of self-renewal capacity or defects in homing.21,22 As a result, some degree of potentially toxic conditioning with chemotherapy or irradiation is required in clinical settings where corrected HSCs and their progeny do not have a competitive advantage.
Vector production to reach a sufficient titer may pose a challenge. Although adequate for most phase 1 or 2 studies, robust manufacturing to support larger trials has yet to be developed. Some specific vectors, such as the complex globin vectors23-25 or vectors containing the cHS4 insulator element,26,27 exhibit lower titers that pose a manufacturing challenge. More research on vector production is needed to advance the field and eventually meet commercial goals.
Sufficient and stable expression of vector-encoded transgenes is another category of concern because of the vagaries of position effects and the risk of transcriptional inactivation.28-30 Although well documented and extensively studied in murine models,30,31 vector silencing has been less investigated in clinical studies. It is noteworthy that the vast collections of integration sites documented in many trials10,11 do not provide information on vector expression. Furthermore, studies in SCID and WAS may blunt such an analysis because of the selective outgrowth of transgene-expressing cells. In the case of CGD, where such selective pressure on transgene expression does not apply, silencing and methylation of the vector's retroviral promoter were found in several clones as early as 5 months after therapy.32 On the other hand, very prolonged expression of marker genes, with no evidence for significant silencing, has been documented in early human clinical and nonhuman primate studies using both γ-retroviral and lentiviral vectors.33-35 Silencing may be more problematic in murine cells, which have evolved mechanisms for inactivation of the huge proviral load integrated into the murine endogenous genome, but occurs in human cells as well.
The toxicities associated with the γ-retroviral transduction of HSCs are the consequence of the semirandom pattern of retroviral integration and the presence of strong enhancers in the proviral LTR,36-38 resulting in obligatory insertional mutagenesis. Activation of proto-oncogenes in the genomic neighborhood is the most dreaded consequence. The direst outcome, frank leukemic transformation, has been dramatically illustrated in X-SCID and WAS, where so far 5 of 20 and 1 of 10 patients, respectively, have developed clonal T-cell leukemias.39,40 A surprising feature of these clonal transformations, all linked to the integration of an LTR-driven γ-retroviral vector in the vicinity of an oncogene, is the striking involvement in all but one case of the LMO-2 gene (Table 2).41,42 This gene is expressed in early hematopoietic progenitors (where it is therefore accessible to the retroviral pre-integration complex) and normally silenced on hematopoietic differentiation, unless the LTR prevents this from occurring,43 which may result in the activation of an HSC-like transcription profile in thymocytes and clonal expansion, eventually resulting in full malignant transformation with the acquisition over time of additional chromosomal abnormalities or point mutations in genes, such as Notch.44 Just as striking is the absence of such transformations in patients with ADA-SCID, who also harbor LTR-containing vectors in their T-cell precursors, including occasional integrations in the vicinity of LMO2.10 Marked expansion of a single corrected clone, persisting without malignant transformation for many years, has been documented in an early ADA-SCID gene therapy trial, suggesting that clonal expansion does not irrevocably progress to malignancy.45 The reason for such contrasting outcomes is still unclear and may have to do the nature of the disease, with less profound and rapid expansion of corrected T-cell precursors, or a unique interaction between the transgene and activated LMO2.46
Clonal expansion, myelodysplasias, and transformation
| Patient/disease/transgene . | Relevant vector sequences (references) . | Secondary effect (mo after treatment) . | Genomic insertion sites (transcript status) . | Other genetic alterations (mo after treatment) . | Reference(s) . |
|---|---|---|---|---|---|
| P4/SCID-X1/γC | MFG(B2), Moloney-MLV LTR (8,138) | T-ALL, mature T cell (30) | LMO2 (↑) | Translocation(6,13); CDKN2A deletion | 3,139 |
| P5/SCID-X1/γC | T-ALL, late cortical T cell (34) | LMO2 (↑) | SIL-TAL microdeletion, trisomy 10, Notch mutation (1593F/S) | 139 | |
| P7/SCID-X1/γC | T-ALL, late cortical T cell (68) | CCND2 (↑) | CDKN2A deletion | 42,139 | |
| P10/SCID-X1/γC | T-ALL, late cortical T cell (33) | LMO2 (↑), BMI1 (↑) | Notch mutation (1707A/P) | 42,139 | |
| P1/X-CGD/gp91phox | SFFV LTR (9,16) | Multiple predominant progenitor cell clones (5), subsequent oligoclonal hematopoiesis, monosomy 7 (21), MDS (27) | MDS1-EVI1 (↑), PRDM16 (=), SETBP1 (↑) | CpG methylation in promoter of the viral LTR (9); CDKN2B and p15INK4B hypermethylation; phosphorylation of H2AX and DNA double-strand breaks (27) | 16,32 |
| P2/X-CGD/gp91phox | Multiple predominant progenitor cell clones (5), subsequent oligoclonal hematopoiesis, monosomy 7 (33), MDS (43) | MDS1-EVI1 (↑), PRDM16 (↑) | CpG methylation in promoter of the viral LTR (15); CDKN2B and p15INK4B hypermethylation; phosphorylation of H2AX and DNA double-strand breaks (43) | 16,32 | |
| P8/SCID-X1/γC | MFG, Moloney-MLV LTR (8,140) | T-ALL (24) | LMO2 (↑) | Notch1 mutation (gain-of-function, 1559R/P), CDKN2A deletion, TCRb/STIL-TAL1 translocation | 41 |
| P2/Thalassemia/β(T87Q)-globin | ΔU3 HIV LTR + 2xcHS4 insulators (24,141) | Dominant, myeloid-biased cell clone | HMGA2 (↑) | Vector rearrangement; transcriptional activation of HMGA2 in erythroid cells with increased expression of a truncated HMGA2 mRNA insensitive to degradation by let-7 micro-RNAs | 24 |
| Patient/disease/transgene . | Relevant vector sequences (references) . | Secondary effect (mo after treatment) . | Genomic insertion sites (transcript status) . | Other genetic alterations (mo after treatment) . | Reference(s) . |
|---|---|---|---|---|---|
| P4/SCID-X1/γC | MFG(B2), Moloney-MLV LTR (8,138) | T-ALL, mature T cell (30) | LMO2 (↑) | Translocation(6,13); CDKN2A deletion | 3,139 |
| P5/SCID-X1/γC | T-ALL, late cortical T cell (34) | LMO2 (↑) | SIL-TAL microdeletion, trisomy 10, Notch mutation (1593F/S) | 139 | |
| P7/SCID-X1/γC | T-ALL, late cortical T cell (68) | CCND2 (↑) | CDKN2A deletion | 42,139 | |
| P10/SCID-X1/γC | T-ALL, late cortical T cell (33) | LMO2 (↑), BMI1 (↑) | Notch mutation (1707A/P) | 42,139 | |
| P1/X-CGD/gp91phox | SFFV LTR (9,16) | Multiple predominant progenitor cell clones (5), subsequent oligoclonal hematopoiesis, monosomy 7 (21), MDS (27) | MDS1-EVI1 (↑), PRDM16 (=), SETBP1 (↑) | CpG methylation in promoter of the viral LTR (9); CDKN2B and p15INK4B hypermethylation; phosphorylation of H2AX and DNA double-strand breaks (27) | 16,32 |
| P2/X-CGD/gp91phox | Multiple predominant progenitor cell clones (5), subsequent oligoclonal hematopoiesis, monosomy 7 (33), MDS (43) | MDS1-EVI1 (↑), PRDM16 (↑) | CpG methylation in promoter of the viral LTR (15); CDKN2B and p15INK4B hypermethylation; phosphorylation of H2AX and DNA double-strand breaks (43) | 16,32 | |
| P8/SCID-X1/γC | MFG, Moloney-MLV LTR (8,140) | T-ALL (24) | LMO2 (↑) | Notch1 mutation (gain-of-function, 1559R/P), CDKN2A deletion, TCRb/STIL-TAL1 translocation | 41 |
| P2/Thalassemia/β(T87Q)-globin | ΔU3 HIV LTR + 2xcHS4 insulators (24,141) | Dominant, myeloid-biased cell clone | HMGA2 (↑) | Vector rearrangement; transcriptional activation of HMGA2 in erythroid cells with increased expression of a truncated HMGA2 mRNA insensitive to degradation by let-7 micro-RNAs | 24 |
The anticipation that malignant transformation after HSC gene therapy would be limited to SCID patients receiving cells engineered to overexpression, a growth-promoting gene, such as the common γ-cytokine receptor and resulting from a unique interaction with insertions activating LMO2, was quashed by subsequent reports indicating that insertional mutagenesis after HSC gene transfer using γ-retroviral was a universal risk. The lack of events in the earlier clinical trials and large animal studies may have resulted from exceedingly low HSC gene transfer efficiency and lack of prolonged follow-up. Nonhuman primates and serially transplanted mice receiving HSCs transduced with γ-retroviral vectors carrying only marker genes developed clonal over-representation or overt myeloid and lymphoid leukemias, with LTR activation of a stereotypical group of proto-oncogenes, most strikingly MDS1/EVI1.47-50 Two patients with CGD receiving corrected CD34+ cells developed first clonal expansion of myeloid cells with vector insertions activating the MDS1/EVI1 or the related PRDM16 gene loci, and then clonal myelodysplasia and marrow failure, with acquisition of an additional monosomy 7 abnormality in the malignant clone in both patients16,32 (Table 2).
The use of LTR-driven γ-retroviral vectors to modify HSCs is thus all but over (with the possible exception of ADA deficiency, which has been remarkably devoid of malignant complications to date). New approaches are needed. The next paths to HSC gene therapy point in 3 directions, pursuing either new designs of randomly integrating viral vectors, targeted gene delivery strategies, or the use of reprogrammed stem cells. Each one of these avenues shows great promise but also distinctive, real challenges.
New retroviral vector designs
New retroviral vector types and vector designs, using a “self-inactivating” (SIN) vector modification of γ-retroviral vectors,51 lentiviral vectors,52 and lineage-restricted vectors,23 are now entering the clinic (Table 1). The first fundamental alteration to the first-generation design (Figure 1A) was the elimination of strong promoter/enhancer elements in integrated proviral LTRs via deletion of the LTR enhancer/promoter region from the 3′ end of the vector, which on proviral integration replaces the 5′ LTR. This SIN design then requires the incorporation of an internal promoter to drive transgene expression (Figure 1B). Hardly a new technique,51 this γ-retroviral vector design is now in use in an X-SCID clinical trial (Table 1). Because of concerns regarding recombination with endogenous HIV, this SIN vector design has been adopted from the get-go in later vectors derived from HIV-152 and foamy viruses53 (Figure 1C).
In addition, HIV-derived vectors may possess a safety advantage over those derived from MLV because of their natural propensity to integrate all along transcription units without preference for promoter regions, in contrast to LTR-driven MLV-derived vectors, as documented both in cell lines and in predictive large animal models.36-38,54-56 These differences in integration patterns have now been verified in human clinical trials via large-scale insertion site analyses.11,17,57,58 New studies using SIN γ-retroviral vectors will be interesting to compare in this regard. Common integration sites detected after HSC lentiviral transduction and transplantation are located throughout large genomic regions and appear to result from integration biases associated with the additional factor of in vivo clonal selection and expansion via proto-oncogene activation documented with γ-retroviral HSC gene transfer.57,59 A number of reviews discuss recent insights into proviral integration into the genome, made possible by high throughput retrieval of integration sites and next-generation sequencing.60-63 The most commonly used lentiviral vectors typically harbor ubiquitous internal promoters to drive transgene expression, such as that of human phosphoglycerate kinase or elongation factor-1α (EF-1α). These are not strong promoters, but they appear adequate for correction of enzymopathies, for which modest amounts of transgene product are therapeutic (on the order of femtograms protein per cell).64 Whether phosphoglycerate kinase, EF-1α, or WAS promoter-driven vectors will prove to be sufficient to redress defects in structural proteins or signaling molecules or receptors is yet to be determined. A concern is that the use of stronger, ubiquitous polII promoter/enhancers will increase the risk of trans-activating neighboring genes and malignant transformation back toward the level of genotoxicity encountered with intact LTR regulatory elements. However, there is evidence that enhancers located within a vector are less prone to activation of neighboring genes than the same enhancer contained within an LTR.65 Expression of miRNAs and shRNAs also require robust expression levels,66,67 but the polIII promoters they use may pose a lesser risk of deregulating endogenous gene expression than polII promoters. RNA-based anti-HIV moieties composing an shRNA to tat/rev, a TAR decoy, and an anti-CCR5 ribozyme were recently evaluated after lentiviral-mediated gene transfer to autologous CD34+ cells in subjects with AIDS-related lymphoma.68 Whereas the gene-marking levels were approximately 2 logs lower than in the ALD study, well below levels expected to provide a therapeutic benefit, sustained expression of the shRNA was detected for up to 24 months in one of the patients, without discernable toxicity. For the most common inherited blood disorders, including the thalassemias and sickle cell anemia, high-level globin chain expression (on the order of picograms per cell)64 requires the vectors to incorporate powerful erythroid-specific enhancers (Figure 1D),23 reviewed elsewhere.69 Here the safety concern posed by inclusion of powerful elements is in part mitigated by their tissue specificity, limiting the probability that an adjacent oncogene would be trans-activated in nonerythroid cells.43,64 A single patient treated with such a vector has been followed for more than 3 years.24 This subject, afflicted with HbE thalassemia, showed sustained expression of the vector-encoded globin starting 5 to 6 months after transplantation, most of which could be attributed to a single expanded clone. This patient is currently leukemia-free despite the prolonged clonal expansion and continues to produce an additional 2 to 3 grams per dL of hemoglobin comprising the vector-encoded β(T37Q)–globin chain. The proviral insertion in this clone resulted in aberrant splicing and dysregulated expression of the HMGA2 gene. A recent murine study links HMGA2 overexpression to clonal myeloid expansion, without leukemic transformation.70 This example serves as a cautionary note regarding the risk for clonal expansion with any integrating vector, even those without strong constitutive enhancers.
Powerful enhancers, especially nonspecific ones, would probably require to be flanked by genetic elements with enhancer-promoter blocking activity.71,72 The optimization use of such elements, however, still remains elusive,73,74 their utility is unproven in human or relevant large animal models, and inclusion in vectors may even precipitate mutagenic events, as demonstrated by the alternative splicing from HMGA2 to the cHS4 chicken insulator core element in the aforementioned expanded clone.24 Additional genetic switches and posttranscriptional regulatory mechanisms75 may add a further layer of control to these various vector designs.
Several other types of integrating retroviruses are being developed as potential gene therapy vectors for HSCs but are much less far along in development and have not yet been used in clinical trials. The human foamy virus has not been associated with disease in any species, has a broad target cell range, and efficiently transduces hematopoietic cells.76 Its integration profile is remarkably random, and it has been used to phenotypically correct CD18 integrin deficiency in a canine model of leukocyte adhesion deficiency.77 The avian sarcoma leucosis virus has also been developed and tested in a nonhuman primate HSC transplantation model. It also has a relatively random integration pattern and has the advantage that the LTR promoter/enhancer is completely inactive in mammalian cells.78
The inclusion of suicide genes in vectors, which would allow ablation of vector-containing cells in the context of an adverse event, has been little investigated in HSCs compared with other cell types, but new studies are exploring their efficacy in preclinical models. To this end, a number of new highly effective suicide genes have been reported,79,80 including the human-derived, dimerizable caspase-9 gene.81 In sum, all of the vector designs shown in Figure 1B through D are expected to reduce the risk of insertional oncogenesis relative to that of LTR-driven γ-retroviral vectors. A number of preclinical models have been developed to try to assess genotoxic risk qualitatively or quantitatively before new vectors are used clinically. These include in vitro immortalization of murine myeloid progenitor cells,82-84 serial transplantation in a murine model,50 transplantation of transduced HSCs from genetically tumor-prone mouse strains,85 or long-term follow-up of nonhuman primates.56 It is reassuring that most of these models come to similar conclusions on the relative genotoxicity of different vector backbones. However, the proof can only come from clinical studies, which will take several more years to come to fruition. It is noteworthy that the vector configurations shown in Figure 1B through D are already undergoing clinical testing (Table 1).
Targeted gene delivery
The aforementioned strategies all continue to rely on semirandom integration of the vector provirus into the HSC genome and, as such, will never be free of genotoxic risk. An alternative goal is the targeting of transgene delivery to a predetermined chromosomal location, or the repair of a mutated locus, greatly decreasing the risk of insertional mutagenesis. Targeted gene delivery and gene repair would be optimal if clinically relevant targeting efficiencies can be achieved without off-target genotoxicity or immediate toxicity to transduced cells.
The gold standard for targeted gene delivery is homologous recombination (HR). HR is a DNA repair mechanism that has been successfully used to repair mutated genes and is therefore applicable in principle to cell-based therapies of monogenic diseases.86 Gene targeting by HR requires the use of homologous DNA surrounding the targeted site, usually delivered as plasmid DNA. Introducing large amounts of plasmid DNA into target cells is inefficient and toxic and has thus posed a major challenge in HSCs. The efficiency of DNA entry and of HR can be increased with the use of adenoviral87 and adeno-associated virus vectors,88,89 but these vector types are not well suited for use in HSCs. Another technique to promote specific HR uses triplex-forming oligonucleotides that bind the major groove of duplex DNA,90 which are coupled to a donor DNA sequence. Using nanoparticles for intracellular oligonucleotide delivery, this approach has been shown to target the endogenous β-globin locus in human CD34+ cells, resulting in levels of globin gene modification in the range of 0.5% to 1.0%.91
The efficiency of HR versus nonhomologous recombination can be increased by the introduction of DNA double-strand breaks at the targeted site using an endonuclease.92 This requires the transient expression of an endonuclease, which can be directed to a specific sequence using modular zinc finger proteins,86 homing endonucleases,93 or transcription activator-like effectors derived from phytopathogenic bacteria.94 Zinc finger nucleases were recently shown to afford remarkable targeting frequencies at the CCR5 locus, disrupting an average 17% of all CCR5 loci in CD34+ cord blood cells, with retained ability to engraft immunodeficient mice and demonstration of engrafted human CCR5-disrupted cells resistant to HIV infection.95 Significant questions remain regarding the efficiency of targeting in bona fide HSCs, and the risk of inflicting off-target effects, which may result in translocations or occult genotoxicity.96 The ability to accurately predict and monitor the off-target effects of modified endonucleases is still an open question.97,98
Gene targeting via HR in HSCs has lagged behind other target cell types because of the challenges inherent in efficiently and nontoxically introducing the endonuclease and corrective targeting constructs into these fragile and rare cells unable to be cloned or effectively expanded ex vivo. However, there has been recent progress using nonintegrating lentiviral vectors or optimized nucleofection.95,99 Off-target genotoxicity and efficiency of long-term HSC correction will be difficult to assess in xenograft models and will require both large-animals studies and pilot clinical trials in a patient population with sufficiently serious disease complications to justify introduction of these potentially risky approaches.
New cell types for HSC engineering
Current strategies to genetically engineer HSCs are confined by our inability to expand or subclone genetically modified HSCs, whether adult or cord blood-derived. Whether this is the result of properties inherent to HSCs or lack of knowledge regarding appropriate culture conditions, any strategy for mitigation of genotoxicity dependent on screening of vector insertion sites before cell administration is not feasible at this time. Recent advances in pluripotent stem cell technology may, however, transform the face of stem cell engineering, allowing much better characterization of corrected cells before clinical use. The ground-breaking discovery of Yamanaka, who successfully reprogrammed mouse fibroblasts to a pluripotent state similar to that of embryonic stem cells after γRV-mediated introduction of the transcription factors Oct4, Sox2, Klf4, and c-myc,100 opens up new prospects for therapeutic stem cell engineering. The feasibility of expanding pluripotent stem cells without compromising their stem cell properties makes it possible to subclone and select genetically modified cells, as well as to perform extensive efficacy and safety testing in the selected clonal derivatives (Figure 2). Thus, relatively inefficient techniques, such as classic HR, which are inapplicable to HSCs because of their inefficiency, now become relevant. These concepts were dramatically illustrated by Hanna et al in a mouse model of sickle cell anemia.101 In this study, the βS-globin gene was corrected by HR in a fibroblast obtained from a humanized sickle cell transgenic mouse, which was then reprogrammed to a pluripotent state by retroviral transduction100 and subjected to in vitro directed hematopoietic differentiation in the presence of HoxB4 protein. Transplantation of the specified hematopoietic cells did not achieve full hematopoietic reconstitution but effectively blunted the sickle cell syndrome.101
Evolving paradigms in HSC engineering. (A) Current strategies are restricted by the use of nonclonable adult HSCs. (B) The advent of patient-specific pluripotent stem cells may open new strategies for genetic engineering and biosafety testing.
Evolving paradigms in HSC engineering. (A) Current strategies are restricted by the use of nonclonable adult HSCs. (B) The advent of patient-specific pluripotent stem cells may open new strategies for genetic engineering and biosafety testing.
Patient-specific induced pluripotent stem (iPS) cells can be generated from various cell types obtained from patients with inherited or acquired disorders, using a range of techniques.102,103 Use of nonintegrating or excisable vectors for generation of iPS cells may avoid issues of insertional mutagenesis and incomplete silencing of reprogramming factors. However, new genetic material must be permanently introduced to correct the underlying disease mutation. Recognizing that iPS-like cells can arise by LV-mediated insertional mutagenesis alone,104 one approach is to pursue targeted correction, via engineered zinc finger nucleases,105 bacterial artificial chromosomes,106 and adeno-associated virus-mediated HR.88 An alternative to HR and its enhanced variations is to screen iPS clones for the integration of lentiviral or other vectors into putative genomic “safe harbors” (ie, sites that sustain transgene expression without interfering with endogenous gene expression).107,108 This capitalizes on the high efficiency of lentiviral transduction and their lack of nonspecific occult genotoxicity but is potentially constrained by imperfect knowledge of all possible mechanisms by which integrated foreign DNA can dysregulate gene expression.
The ability to generate patient-specific iPS cells and correct their abnormalities via genetic repair or transgene delivery at well-characterized “safe harbor” sites108 does not imply that the use of pluripotent stem cells as a source of HSCs is ready for implementation or that it may ever become suitable for human application. Several major questions need to be further investigated: how to best generate iPS cells efficiently with minimal genotoxicity imparted through the reprogramming process; how to identify and qualify iPS clones that are suitable for clinical investigation, which addresses the genetic, epigenetic, tumorigenic, and differentiation potential of individual iPS clones; how to genetically engineer iPS clones effectively but without adding any genotoxic insults through the repair process; how to generate engraftable HSC-like cells capable of full and durable hematopoietic reconstitution in transplanted recipients; and how to scale up the differentiation culture processes and ensure the depletion of cells with teratoma formation potential. Some of these concerns apply to the use of pluripotent stem cells or reprogrammed cells in general, whereas others specifically relate to their potential use for hematopoietic applications.
Recent studies have documented frequent genetic alterations in human ES and iPS cells, including point mutations (some affecting oncogenes), deletions, and gene duplications.109-111 These occur independently of the reprogramming vectors and are thus distinct from the problems related to insertional mutagenesis. These events may be in part linked to the reprogramming phase, which is known to be enhanced in the absence of p53.112-114 Successful reprogramming could even require accumulation of genetic and/or epigenetic alterations in cells undergoing extended cell culture, including pluripotent stem cells, and the low efficiency of reprogramming may result from a requirement for rare permissive mutations.115-117 In one recent report comparing the genome sequence of fibroblasts before and after reprogramming,118 several mutations found in the iPS cells could be traced back to the original fibroblast; however, others arose during the reprogramming period. No further genomic alterations were detected after further clonal expansion, suggesting the unique susceptibility or requirement for genomic changes during reprogramming. More studies are needed to better gauge the intrinsic genotoxicity of reprogramming, which may be unavoidable even if reprogramming were to be induced by chemical or other means that avoid the use of integrating vectors. The immunogenicity of iPS-derived cells may also cause concern, although currently available information is limited to the pluripotent stem cells themselves.119
The generation of engraftable adult HSCs from human ES and iPS cells remains elusive to date and probably represents the single largest hurdle to use of pluripotent or reprogrammed cells for hematologic applications. The first hematopoietic cells arise in the primitive streak of the embryo, yielding a distinctive “primitive” hematopoiesis120 that cannot reconstitute adult hosts, and produces, for example, erythroid cells with embryonic hemoglobins that would be unable to function optimally for oxygen delivery in postnatal life. The immediate precursors of “definitive” HSCs are arterial endothelial cells, which generate HSCs capable of long-term multilineage repopulation of adult hosts beginning in the dorsal aorta of the aorta-gonad-mesonephros region and the chorioallantoic vessels of the placenta.121-126 It is these early CD34+, c-kit+, CD41+ HSCs that migrate to the yolk sac and the fetal liver, where they vastly expand before relocating to the bone marrow around birth.120 To date, candidate HSCs derived from human ES and iPS cells by and large fail to engraft and reconstitute irradiated adult recipients.127-130 Even production of engrafting HSCs from murine ES cells, studied intensively for more than 20 years, is extremely inefficient. Only the ectopic expression of the transcription factor HoxB4 in the hematopoietic progeny of murine ESCs has resulted in long-term efficient in vivo engraftment.131 Ectopic expression of HoxB4 has been shown to result in abnormal myeloid/lymphoid ratios in mice, and leukemogenesis in dogs and monkeys, suggesting that this approach to driving hematopoiesis from pluripotent ES or IPS cells is not clinically relevant.84,132,133
Another recent approach, bypassing the need for iPS cells and the obstacles to generating HSCs from embryonic-type cells, consists of direct reprogramming of skin cells to a multipotent progenitor stage via introduction of a single transcription factor, Oct4.134 Unlike ES and iPS-derived hematopoietic cells, Oct4-reprogrammed progenitor cells possess desirable traits, such as the expression of adult globin genes on erythroid differentiation, and robust albeit short-term myeloid engraftment potential in immunodeficient mice. The exact nature and therapeutic potential of these cells are presently unknown, but these findings point to tantalizing discoveries that may come out of reprogramming and trans-differentiation research.135 Another game-changing advance would entail the ability to reprogram human adult HSCs to an expandable state without diminishing their long-term self-renewal properties and their safety, a goal that has remained elusive to date.
HSC engineering at a crossroads
HSC engineering is now at a crossroads. HSC gene therapy has proven benefits in patients with severe immunodeficiencies, but the MLV-derived LTR-driven vectors used in initial clinical trials pose too great a risk of genotoxicity for further clinical development. As their clinical use comes to an end, with the possible exception of ADA deficiency, the first chapter of HSC gene therapy is now closed. New approaches are needed, and their development will greatly benefit from important lessons learned in the early age of gene therapy. Clinical experience with LTR enhancer-deleted MLV vector- or lentiviral vector-transduced HSCs is not mature enough for meaningful risk assessment, although results from animal models and in vitro transformation assays suggest that either of these vector classes will be significantly less genotoxic and thus safer. Any integrating vector can integrate near and activate oncogenes, but removal of strong ubiquitous enhancers from both self-inactivating MLV vectors and lentiviral vectors will greatly decrease the latter risk. However, other genetic effects, such as abnormal splicing events,24 or inactivation of tumor suppressor genes, may still occur. Lentiviral vectors, as well as several novel types of vectors in preclinical development, including those derived from human foamy virus or avian sarcoma leucosis virus, have integration profiles that differ subtly in the targeting of genes or their promoter region, which may result in significantly different safety profiles.36,78,136 Lentiviral vectors encoding ubiquitous promoters of moderate strength afford adequate titers and express transgenes at levels that seem to be appropriate for enzymatic deficiencies. The design of vectors that are better suited for conditions requiring higher protein expression is at the present less well defined.
Targeted gene delivery approaches, including the use of zinc finger nucleases, meganucleases, transcription activator-like effector nucleases, and triplex-forming oligonucleotides, have made great advances. Their targeting frequencies, albeit 1 to 2 logs lower than retroviral-mediated gene transfer efficiency, are on the way to reaching clinically relevant values.
Somatic cell reprogramming opens the door to many genetic engineering approaches, including screening for retroviral integrations in potential genomic safe harbors108 and targeted gene delivery, including nuclease-based approaches and adeno-associated virus-mediated homologous recombination. The advent of iPS cells is far from clinically relevant and poses a number of fascinating biologic questions, spanning a broad range of issues that concern the epigenetic and genetic status of iPS cells, their differentiation potential, and their propensity to transform, whether they have been genetically modified or not. In particular, the generation of human HSCs from ES or iPS cells remains an enigma, one that will hopefully soon be resolved.
Genotoxicities remain a fundamental concern in all the aforementioned approaches: insertional mutagenesis in the case of retroviral-mediated gene transfer, off-target effects when using nucleases to induce double-strand breaks to enhance targeted gene delivery, genetic alterations incurred during reprogramming to a pluripotent state, and genetic alterations arising throughout extended cell culture. Inclusion of suicide genes137 allowing in vivo ablation of dangerous clones may be worthwhile and is under preclinical development.
Prospects
The first 2 decades of HSC gene therapy have been rich in lessons, providing both strong support for the therapeutic potential of this approach and sobering lessons on the shortcomings of the genetic engineering of stem cells. As the chapter on LTR-driven vectors comes to a close, several new chapters are already being written. The immediate future will evaluate SIN-γRVs, SIN-LVs, and lineage-restricted vectors, all of which should reduce the risk of trans-activating proto-oncogenes after semirandom integrations. Next, targeted gene delivery systems have the potential to further reduce the risk of integrating vectors at undesirable chromosomal locations. Later, if pluripotent stem cells fulfill their promise for the generation of HSCs and if the genotoxicity issues of their own prove not to be prohibitive, genetically corrected cells in which vector integration or gene repair can be fully ascertained before cell infusion will become available. Despite the significant challenges, at least one of these new directions will eventually lead to safe and effective HSC therapies for hereditary and acquired disorders. HSC engineering remains one of the most tantalizing medical research objectives for the 21st century.
Acknowledgments
The authors thank Jason Plotkin for help with figures.
I.R. and M.S. were supported by the National Institutes of Health (grants HL053750, CA59350, and CA08748), the Experimental Therapeutics Center at Memorial Sloan-Kettering Cancer Center, the Niarchos Foundation, the Leonardo Giambrone Foundation, the Cooley's Anemia Foundation, and NYSTEM.
National Institutes of Health
Authorship
Contribution: I.R., C.E.D., and M.S. wrote the manuscript.
Conflict-of-interest disclosure: M.S. holds patents on globin gene transfer and chimeric antigen receptors for immune engineering. The remaining authors declare no competing financial interests.
Correspondence: Michel Sadelain, Center for Cell Engineering, Molecular Pharmacology and Chemistry Program, Memorial Sloan-Kettering Cancer Center, Box 182, 1275 York Ave, New York, NY 10065; e-mail: m-sadelain@ski.mskcc.org.