In this issue of Blood, Sampath and colleagues provide an important missing link in how microRNAs (miRs) can be silenced in chronic lymphocytic leukemia (CLL): histone deacetylases (HDACs) that are overexpressed in CLL block critical miRs in the malignant B cell resulting in pro-survival signals. Thus, HDAC inhibition is an attractive new therapeutic strategy in CLL.1
A disrupted apoptotic machinery, the main pathogenic mechanism in CLL, results in an enormous accumulation of malignant B cells in this most common leukemia. MicroRNAs that are globally down-regulated in CLL can mediate antiapoptotic signals via up-regulation of critical molecules like Mcl-1 and Bcl-2.2 Critical players in this match for life and death in the microRNA field are, among others, miR-15a, miR-16, and miR-29b.3 The story seemed straightforward because at least miR-15a and miR-16 are located at the 13q14 locus that also represents the locus most frequently deleted in CLL.4 Nature is much more complicated than initially thought: more than one-third of CLL cases have an intact chromosome 13 and some of these still show down-regulation of miR15a and miR16 in addition to other deregulated microRNAs. Now, a new pathogenic mechanism has been identified: epigenetic modulation of microRNA expression. One responsible protagonist in this death drama has also now been identified: HDACs.
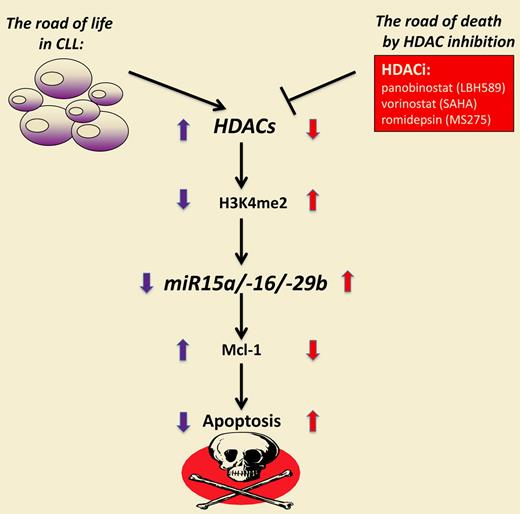
HDAC inhibition restores expression of miRs and triggers cell death in CLL.
HDAC inhibition restores expression of miRs and triggers cell death in CLL.
HDACs are enzymes involved in remodeling of chromatin by deacetylating the lysine residues and they play a pivotal role in epigenetic regulation of gene expression. In tumors, posttranslational modification of histones and nonhistone proteins by acetylation seems to be very critical. An aberrant activity of HDACs has been documented in several types of cancers and HDACs have, therefore, emerged as an attractive therapeutic target in recent years.
Here now is the missing link that researchers in the field were waiting for: CLL cells express significant amounts of HDACs that mediate silencing of critical microRNAs like miR-15a and miR-16. And the story can also be reversed: inhibition of HDACs induces expression of the critical microRNAs, causing a decrease of the pro-survival protein Mcl-1, that is, cell death (see figure). HDAC inhibition results in increased acetylation of histones and triggers the accumulation of H3K4me2 at the miR15a/-16 promoters. Of interest, different members of this class of therapeutics including LBH589, vorinostat, and romidepsin also produced the same effects.
But there are still secrets in this matter of life and death in CLL. What about the other two-thirds of cases, that is, the majority of samples examined, where HDAC inhibition did not have any effect on the expression of these critical microRNAs? Why haven't we heard more about HDAC inhibitors in CLL after initial trials with epigenetic drugs like depsipeptide and others?5 Should in vitro testing first identify eligible patients who are able to reverse epigenetic silencing of miRs by HDAC inhibitors before evaluating these drugs in clinical trials?
Besides histone modifications by deacetylation, DNA methylation also seems to be critical in CLL pathogenesis. The first evidence for epigenetic regulation of miRNA was derived from cell line studies using demethylating drugs to induce transcriptional reactivation of miRNAs.6,7 An important role of DNA hypermethylation for down-regulation of miRNAs in CLL was suggested by Pallasch and coworkers.8 Nevertheless, a systematic genome-wide profiling of DNA methylation changes at putative miRNA promoters in CLL compared with healthy B cells will be necessary to establish the definitive role of methylation in CLL pathogenesis. It will be of clinical interest to answer the question whether demethylating agents like 5-aza-2′deoxycytidine can result in derepression of epigenetically silenced miRNA promoters as an alternative approach to HDAC inhibitors. After tyrosine kinase inhibitors like the PI3K inhibitor CAL101 and the Bruton tyrosine kinase (BTK) inhibitor PCI-32765 have recently entered the scene in the fight against CLL, now the battlefield seems to be opened for other weapons hitting the Achilles heel of life and death at another angle.9,10 Epigenetic drugs including HDAC inhibitors should now be carefully explored in well-defined, molecularly characterized groups of patients suffering from CLL within controlled clinical trials, hopefully broadening the armamentarium against this still incurable disease.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal