Abstract
We investigated a recent (January 1999 to December 2009) cohort of 95 elderly Hodgkin lymphoma subjects. At diagnosis, median age was 67 years (range, 60-89 years), whereas 61% had significant comorbidity, 26% were unfit, 17% had a geriatric syndrome, and 13% had loss of activities of daily living. Overall response rate to therapy was 85%, whereas incidence of bleomycin lung toxicity was 32% (with associated mortality rate, 25%). With 66-month median follow-up, 2-year and 5-year overall survival were 73% and 58%, respectively (advanced-stage, 63% and 46%, respectively). Most International Prognostic Score factors were not prognostic on univariate analyses, whereas Cox multivariate regression identified 2 risk factors associated with inferior overall survival: (1) age more than 70 years (2.24; 95% CI, 1.16-4.33, P = .02) and (2) loss of activities of daily living (2.71; 95% CI, 1.07-6.84, P = .04). Furthermore, a novel survival model based on number of these risk factors (0, 1, or 2) showed differential 2-year OS of 83%, 70%, and 13%, respectively (P < .0001) and 5-year OS of 73%, 51%, and 0%, respectively (P < .0001).
Introduction
Survival rates for elderly Hodgkin lymphoma (eHL), typically defined as more than equal to 60 years of age, are disproportionately inferior compared with younger patients.1 Studies in the 1970s to the 1990s established 5-year progression-free survival (PFS) or freedom from treatment failure rates for advanced-stage eHL of 30% to 45% with 5-year overall survival (OS) rates of 40% to 55%.2-8 There are limited data examining eHL outcomes in the modern era.
Furthermore, a paucity of prognostic data exist for eHL, while the potential impact of functional status on survival is largely unexplored, including recent reports.9 The most established prognostic tool in HL is the International Prognostic Score (IPS), which uses 7 adverse clinical prognostic factors, including age older than 45 years, to predict outcome.10 However, only 9% of patients in that analysis were older than 55 years, whereas none older than 65 years were included (Volker Diehl, personal oral communication, October 2010).
Methods
We conducted a multicenter retrospective analysis of patients consecutively diagnosed and treated with eHL at 5 medical centers between January 1999 and December 2009; the study was approved by each institution's institutional review board. All cases were pathologically confirmed by expert hematopathologists.11 Staging and therapy were at the treating physician's discretion. Of 111 cases, 95 had complete data and were entered into a centralized database.
Functional status
Functional status was obtained retrospectively. Comorbidity was determined using the Cumulative Illness Rating Scale (CIRS), which assesses basic chronic medical illnesses taking into account the severity of each and has been revised and validated to reflect common geriatric problems (CIRS-G).12,13 The presence of geriatric syndromes at diagnosis (dementia, delirium, depression, osteoporosis, incontinence, falls, failure to thrive, and/or neglect/abuse) and loss of activities of daily living (ADL; ie, loss in: activity of bathing, dressing, toileting, transferring, feeding, and/or continence) were also documented. “Fit” was defined as no loss of ADLs, less than 3 grade 3 CIRS-G comorbidities, no grade 4 CIRS-G, and no geriatric syndrome at diagnosis.14,15
Statistical analysis
PFS (time from date of eHL diagnosis to death or disease relapse/progression) and OS (time from date of eHL diagnosis to death or last follow-up) were per Kaplan-Meier method. Survival differences were assessed using the log-rank test. For comparison with a healthy population, instantaneous survival statistics for each matched age from the US Social Security Department database were used; the average of these weights was computed as determined by the proportion of men and women in our population. Univariate associations between clinical/laboratory factors and survival were derived using the Cox proportional hazards model.16 Variables with a P value ≤ .05 in univariate analyses were entered into the multivariate Cox proportional hazards model in a stepwise fashion. Significant factors in multivariate analysis were incorporated into a prognostic model for survival constructed by classification and regression tree analysis. All statistical analyses were via SAS Version 9.2 (SAS Institute).
Results and discussion
Baseline characteristics
Among 95 eHL subjects, the median age was 67 years (range, 60-89 years), with one-third ages 70 to 79 years and 7% age 80 to 89 years. At diagnosis, 21% had history of coronary artery disease and 16% had diabetes. Disease characteristics included 54% B symptoms, 27% performance status 2 to 4, only 4% with bulky disease, 25% with bone marrow involvement, while 20% had other/nonmarrow extranodal disease (most common, bone and lung). The relatively high incidence of B symptoms and low rate of bulky disease are consistent with prior eHL series.1,3,7,8,17,18 Altogether, 64% of patients here had stage 3 or 4, of which 58% had an IPS of 4 to 7. Histology was nodular sclerosis in 47%, mixed cellularity 31%, not otherwise specified 16%, lymphocyte predominant 5%, and lymphocyte depleted 1%. These data confirm prior studies showing eHL more commonly presents with mixed cellularity subtype,3-5 whereas younger patients more frequently have bulky disease at diagnosis supporting that eHL may be biologically distinct.
Functional status
We identified that 61% of patients had a CIRS-G grade 3 or 4 in at least one category, whereas 46% had a cumulative CIRS-G score more than 6. Further, at time of eHL diagnosis, 26% were classified as “not fit,” 17% had presence of a geriatric syndrome, and 13% had loss of ADLs. To our knowledge, this is the first study to examine the prevalence of geriatric syndrome and level of fitness (including ADLs) in eHL.
The presence of comorbidity as a prognostic factor is particularly relevant for older patients. In a population-based study, van Spronsen et al reported that among eHL, 56% had a serious comorbid condition versus 13% in younger patients (P < .0001).19 Levis et al reported results of eHL patients who received lower-intensity chemotherapy20 ; multivariate analysis found that the presence of comorbidity independently correlated with disease-specific survival and OS. Furthermore, disease-specific survival was identical to OS, suggesting ineffective treatment and/or different biology rather than death from non-HL causes.
Treatment
Intensive regimens, such as BEACOPP (escalated or baseline), are too toxic for eHL,21 whereas bleomycin-containing regimens, such as ABVD, are often not tolerated.3-5,22-24 Levis et al analyzed outcomes of patients 65 years of age or older receiving a registry-recommended protocol of ABVD, MOPP, or ABVD/MOPP therapy.4 The 8-year PFS and OS were 41% and 46%, respectively, both significantly inferior compared with patients younger than 65 years; notably, 23% of eHL patients receiving ABVD-based therapy had treatment-related death.
Primary treatment here consisted of: ABVD-based (n = 67), MOPP-based (n = 6), BCVPP (n = 6), ChlVPP (n = 5), radiation alone (n = 4), CHOP (n = 3), hospice (n = 2), BEACOPP (n = 1), and watchful waiting (n = 1). A total of 78% of patients received G-CSF, the majority (63%) of which was pegfilgrastim. Landgren et al showed previously that relative dose intensity more than or equal to 65% was associated with improved survival with MOPP or ABVD-based therapy.18 The relative dose intensity here was 71%. Relative dose intensity did not correlate with survival; however, data were not available for all subjects. The overall response rate among the 92 treated patients was 85% (73% CR). The incidence of bleomycin lung toxicity (BLT) was 32%, which had an associated mortality rate of 25%. Moreover, the incidence of BLT was 38% versus 0% among patients receiving G-CSF versus not, respectively (P = .0001).
Risk factors for BLT include older age, cumulative bleomycin dose, renal insufficiency, pulmonary radiation, underlying lung disease, and tobacco history. G-CSF may increase the incidence of BLT25 ; others have shown an increased incidence of BLT when G-CSF is used during bleomycin-containing chemotherapy with associated mortality rates more than 20%.24 Our data supports this association.
Outcomes
Among older patients entered onto the German Hodgkin Study Group protocols, patients 60 years of age or older had significantly worse freedom from treatment failure than younger patients.3 This difference remained significant with exclusion of events unrelated to HL. A United States Surveillance, Epidemiology, and End Results data analysis by Brenner et al reported increasing survival of all ages of HL, including elderly patients, comparing outcomes from 1980 to 1984 with 2000 to 2004.26 Notably, the 1980 to 1984 survival rates for eHL were exceptionally low (∼ 20%-25%), whereas 2000 to 2004 survival rates remained markedly inferior compared with younger populations.
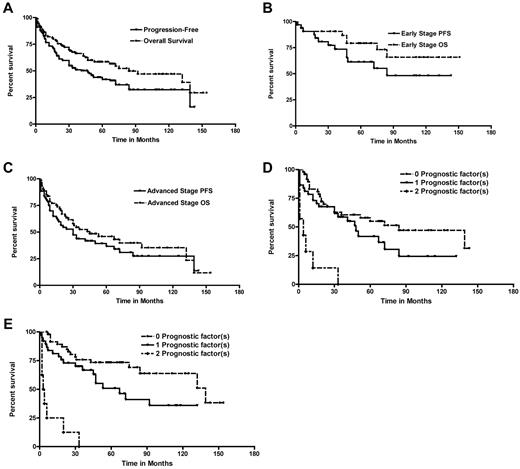
With a median follow-up of 66 months (range, 6-151 months), we documented an overall 5-year PFS and OS of 44% and 58%, respectively. Stage 1 and 2 patients fared better compared with stage 3 or 4 patients (5-year PFS and OS: 61% and 79% for stage 1 or 2 vs 36% and 46% for stage 3 or 4; P = .009 and P = .001, respectively; Figure 1). We also compared the OS of the eHL cohort with an age- and sex-matched healthy population (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Among the 44 eHL patients who died, 35 were as a result of HL (5 from BLT, 1 treatment-related sepsis, 2 cardiac disease, and 1 prostate cancer). Univariate analyses of prognostic factors for survival are noted in Table 1. Interestingly, 4 IPS factors (anemia, white blood cell count, sex, and lymphopenia) were not significant on univariate or multivariate analysis.
Outcomes for eHL. (A). Kaplan-Meier curves of PFS and OS for all eHL patients (n = 95). (B) PFS and OS for early-stage patients. (C) PFS and OS for advanced-stage eHL. Kaplan-Meier curves of (D) PFS and (E) OS for eHL patients based on number of the adverse prognostic factors present (age ≥ 70 years and loss of ADLs). The numbers of patients with 0, 1, or 2 factors at diagnosis were 48, 38, and 9, respectively; increasing number of risk factors portended an increasingly poor survival. A survival model based on the number of adverse factors present (0, 1, or 2) was formed: 2-year PFS, 68% (95% CI, 52%-78%), 68% (95% CI, 50%-80%), and 13% (95% CI, 0%-42%), respectively (P < .001); 2-year OS, 83% (95% CI, 69%-90%), 70% (95% CI, 53%-82%), and 13% (95% CI, 0%-42%), respectively (P < .001); 5-year PFS, 55% (95% CI, 39%-68%), 39% (95% CI, 21%-55%), and 0%, respectively (P < .0001); and 5-year OS, 73% (95% CI, 58%-84%), 51% (95% CI, 32%-67%), and 0%, respectively (P < .0001).
Outcomes for eHL. (A). Kaplan-Meier curves of PFS and OS for all eHL patients (n = 95). (B) PFS and OS for early-stage patients. (C) PFS and OS for advanced-stage eHL. Kaplan-Meier curves of (D) PFS and (E) OS for eHL patients based on number of the adverse prognostic factors present (age ≥ 70 years and loss of ADLs). The numbers of patients with 0, 1, or 2 factors at diagnosis were 48, 38, and 9, respectively; increasing number of risk factors portended an increasingly poor survival. A survival model based on the number of adverse factors present (0, 1, or 2) was formed: 2-year PFS, 68% (95% CI, 52%-78%), 68% (95% CI, 50%-80%), and 13% (95% CI, 0%-42%), respectively (P < .001); 2-year OS, 83% (95% CI, 69%-90%), 70% (95% CI, 53%-82%), and 13% (95% CI, 0%-42%), respectively (P < .001); 5-year PFS, 55% (95% CI, 39%-68%), 39% (95% CI, 21%-55%), and 0%, respectively (P < .0001); and 5-year OS, 73% (95% CI, 58%-84%), 51% (95% CI, 32%-67%), and 0%, respectively (P < .0001).
Patient and disease characteristics with univariate analysis
| Prognostic factor . | PFS . | OS . | ||||
|---|---|---|---|---|---|---|
| HR* . | 95% CI . | P . | HR* . | 95% CI . | P . | |
| Age | ||||||
| Continuous variable | 1.05 | 1.01-1.09 | .009 | 1.07 | 1.02-1.12 | .004 |
| ≥ 70 y vs 60-69 y | 1.99 | 1.16-3.41 | .01 | 2.37 | 1.29-4.36 | .006 |
| Sex (female vs male) | 1.37 | 0.74-2.52 | .32 | 1.34 | 0.67-2.66 | .41 |
| History of CAD | 1.94 | 1.04-3.63 | .04 | 2.20 | 1.14-4.27 | .02 |
| Diabetes | 0.28 | 0.04-2.31 | .24 | 0.33 | 0.04-2.74 | .30 |
| Prior malignancy | 1.51 | 0.86-2.67 | .16 | 1.37 | 0.71-2.65 | .35 |
| No. of medications (> 5 vs < 5) | 1.33 | 0.73-2.45 | .35 | 1.26 | 0.64-2.46 | .50 |
| Presence of B symptoms | 2.32 | 1.31-4.11 | .004 | 3.38 | 1.70-6.72 | .0005 |
| Weight loss (> 10% baseline) | 1.72 | 0.99-2.98 | .055 | 2.01 | 1.09-3.70 | .02 |
| Performance status 3 or 4 (vs 0-2)† | 2.40 | 1.36-4.26 | .003 | 3.76 | 2.02-6.99 | < .0001 |
| CIRS-G | ||||||
| Any score 3 or 4 | 1.94 | 1.09-3.45 | .03 | 2.44 | 1.24-4.79 | .01 |
| > 6 (cumulative) | 1.28 | 0.75-2.18 | .36 | 1.85 | 1.01-3.39 | .047 |
| Loss of any ADLs | 3.61 | 1.79-7.26 | .0003 | 5.61 | 2.71-11.63 | < .0001 |
| Presence of “geriatric” syndrome | 1.47 | 0.77-2.80 | .24 | 1.76 | 0.86-3.59 | .12 |
| Patient fit (no vs yes) | 1.35 | 0.76-2.41 | .31 | 1.57 | 0.82-2.99 | .17 |
| Albumin < 4.0 g/dL | 1.89 | 0.94-3.76 | .07 | 3.57 | 1.45-8.81 | .006 |
| WBC ≥ 15 000/mm3 | 1.89 | 0.45-7.93 | .38 | 1.28 | 0.17-9.40 | .81 |
| Lymphocyte count (< 600/mm3 or < 8% of total WBC) | 1.20 | 0.57-2.53 | .63 | 1.88 | 0.87-4.08 | .11 |
| Hemoglobin < 10.5 g/dL | 1.44 | 0.80-2.58 | .22 | 1.57 | 0.82-2.99 | .17 |
| BM involvement | 2.07 | 1.09-3.93 | .03 | 1.65 | 0.80-3.40 | .17 |
| > 1 EN site | 2.34 | 1.21-4.50 | .01 | 1.74 | 0.80-3.80 | .16 |
| Bulky disease > 7 cm | 0.90 | 0.29-2.80 | .85 | 0.83 | 0.26-2.65 | .75 |
| Stage 3 or 4 (vs 1 or 2)‡ | 2.23 | 1.19-4.17 | .01 | 3.27 | 1.52-7.07 | .003 |
| Cell type | ||||||
| MC | 1.02 | 0.51-2.03 | .96 | 1.21 | 0.55-2.68 | .64 |
| NS | 0.71 | 0.34-1.47 | .36 | 0.79 | 0.34-1.84 | .59 |
| LP | 0.17 | 0.02-1.29 | .07 | 0.28 | 0.04-2.25 | .23 |
| Prognostic factor . | PFS . | OS . | ||||
|---|---|---|---|---|---|---|
| HR* . | 95% CI . | P . | HR* . | 95% CI . | P . | |
| Age | ||||||
| Continuous variable | 1.05 | 1.01-1.09 | .009 | 1.07 | 1.02-1.12 | .004 |
| ≥ 70 y vs 60-69 y | 1.99 | 1.16-3.41 | .01 | 2.37 | 1.29-4.36 | .006 |
| Sex (female vs male) | 1.37 | 0.74-2.52 | .32 | 1.34 | 0.67-2.66 | .41 |
| History of CAD | 1.94 | 1.04-3.63 | .04 | 2.20 | 1.14-4.27 | .02 |
| Diabetes | 0.28 | 0.04-2.31 | .24 | 0.33 | 0.04-2.74 | .30 |
| Prior malignancy | 1.51 | 0.86-2.67 | .16 | 1.37 | 0.71-2.65 | .35 |
| No. of medications (> 5 vs < 5) | 1.33 | 0.73-2.45 | .35 | 1.26 | 0.64-2.46 | .50 |
| Presence of B symptoms | 2.32 | 1.31-4.11 | .004 | 3.38 | 1.70-6.72 | .0005 |
| Weight loss (> 10% baseline) | 1.72 | 0.99-2.98 | .055 | 2.01 | 1.09-3.70 | .02 |
| Performance status 3 or 4 (vs 0-2)† | 2.40 | 1.36-4.26 | .003 | 3.76 | 2.02-6.99 | < .0001 |
| CIRS-G | ||||||
| Any score 3 or 4 | 1.94 | 1.09-3.45 | .03 | 2.44 | 1.24-4.79 | .01 |
| > 6 (cumulative) | 1.28 | 0.75-2.18 | .36 | 1.85 | 1.01-3.39 | .047 |
| Loss of any ADLs | 3.61 | 1.79-7.26 | .0003 | 5.61 | 2.71-11.63 | < .0001 |
| Presence of “geriatric” syndrome | 1.47 | 0.77-2.80 | .24 | 1.76 | 0.86-3.59 | .12 |
| Patient fit (no vs yes) | 1.35 | 0.76-2.41 | .31 | 1.57 | 0.82-2.99 | .17 |
| Albumin < 4.0 g/dL | 1.89 | 0.94-3.76 | .07 | 3.57 | 1.45-8.81 | .006 |
| WBC ≥ 15 000/mm3 | 1.89 | 0.45-7.93 | .38 | 1.28 | 0.17-9.40 | .81 |
| Lymphocyte count (< 600/mm3 or < 8% of total WBC) | 1.20 | 0.57-2.53 | .63 | 1.88 | 0.87-4.08 | .11 |
| Hemoglobin < 10.5 g/dL | 1.44 | 0.80-2.58 | .22 | 1.57 | 0.82-2.99 | .17 |
| BM involvement | 2.07 | 1.09-3.93 | .03 | 1.65 | 0.80-3.40 | .17 |
| > 1 EN site | 2.34 | 1.21-4.50 | .01 | 1.74 | 0.80-3.80 | .16 |
| Bulky disease > 7 cm | 0.90 | 0.29-2.80 | .85 | 0.83 | 0.26-2.65 | .75 |
| Stage 3 or 4 (vs 1 or 2)‡ | 2.23 | 1.19-4.17 | .01 | 3.27 | 1.52-7.07 | .003 |
| Cell type | ||||||
| MC | 1.02 | 0.51-2.03 | .96 | 1.21 | 0.55-2.68 | .64 |
| NS | 0.71 | 0.34-1.47 | .36 | 0.79 | 0.34-1.84 | .59 |
| LP | 0.17 | 0.02-1.29 | .07 | 0.28 | 0.04-2.25 | .23 |
CAD indicates coronary artery disease; WBC, white blood cell count; BM, bone marrow; EN, extranodal; MC, mixed cellularity; NS, nodular sclerosis; CIRS-G, cumulative illness rating scale-geriatrics; y, years; ADL, activities of daily living; and LP, lymphocyte predominant.
HR > 1 indicates a factor with poor prognosis, whereas HR < 1 indicates a factor with favorable prognosis.
PS 2-4 vs 0-1 was not significant.
Stage 4 vs stages 1-3 portended similar prognostic importance as stages 3 or 4 vs stages 1 or 2.
Multivariate analysis and survival model
Few available reports have studied prognostic factors predictive of survival in eHL, and even fewer have examined the relationship between functional status and outcome. Enblad et al, analyzing registry data from 1985 through 1992 for eHL, showed that the IPS did not independently predict outcome.17
On multivariate regression analysis, only 2 factors here were associated with inferior outcomes: (1) age more than or equal to 70 years (PFS: hazard ratio = 1.76, 95% CI, 0.98-3.16, P = .06; and OS: hazard ratio = 2.24, 95% CI, 1.16-4.33, P = .02); and (2) loss of ADLs (PFS: hazard ratio = 2.47, 95% CI, 0.98-6.21, P = .055; and OS: hazard ratio = 2.71, 95% CI, 1.07-6.84, P = .04; supplemental Table 1). Furthermore, these factors remained significant with the 5 subjects with lymphocyte predominant histology excluded (supplemental Tables 2-3). A survival model by classification and regression tree analysis is illustrated in Figure 1. Collectively, these findings highlight the critical impact of functional status on survival the continued modest outcomes of eHL. Prospective trials, including the Study of Hodgkin Lymphoma in the Elderly/Lymphoma Database (SHIELD),27 validating these findings are warranted.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.M.E. designed research, performed research, analyzed data, and wrote the paper; I.H., E.R., C.N., and S.M.S. performed research, analyzed data, and wrote the paper; R.K., B.H., and B.P. performed research; S.S., A.L., J.M.M., S.G., and L.I.G. wrote the paper; and B.J. performed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrew M. Evens, Division of Hematology/Oncology, University of Massachusetts Medical School, 55 Lake Ave North, Worcester, MA 01655; e-mail: andrew.evens@umassmed.edu.