To the editor:
Human blood monocytes are divided into 3 subsets: classical (CD14++CD16−), intermediate (CD14++CD16+), and nonclassical (CD14+CD16++).1 The latter 2 subpopulations produce inflammatory cytokines in response to a wide variety of pattern receptor ligands and are constantly surveying the endothelium for signs of inflammation or damage, whereas classical ones are the main producers of anti-inflammatory IL-10.2 There appears to be a developmental relationship between these cells (from classical by intermediates to nonclassical), and the presence of macrophage colony-stimulating factor (M-CSF) seems to be crucial for their differentiation.3,4 It has been recently shown in the mouse model that antibody-blocking M-CSF receptor (R) depletes the nonclassical monocytes, tissue and tumor-associated macrophages.5 In addition, targeted disruption of the M-CSFR gene resulted in profound mononuclear phagocyte deficiency in mice.6 However, there is no direct evidence that blockade of M-CSF/M-CSFR axis in humans inhibits maturation or survival of any circulating monocyte subpopulation.
In a double-blinded substudy of a phase 1b clinical trial (registration number: NCT00550355 on www.clinicaltrials.gov) we analyzed the absolute number of 3 circulating subpopulations of monocytes from 2 patients with active rheumatoid arthritis (RA) receiving human monoclonal antibodies (mAbs) against M-CSF (PD 0360324 of IgG2 isotype), which prevents M-CSF binding to M-CSFR, or placebo. PD 0360324 is a biologic compound being developed by Pfizer for inflammatory conditions. According to study inclusion criteria, 2 adult patients (female, aged 44, and male, aged 43) diagnosed with RA for 4 and 2 years, respectively, were enrolled and their monocyte subpopulations were monitored. Patients have had insufficient response to methotrexate (MTX) while receiving a stable dose of 25 mg weekly for at least 6 weeks before enrollment (MTX does not influence phenotypic differentiation of circulating monocytes7 ). A 100-mg dose of PD 0360324 or placebo was administered as an intravenous infusion at days 1, 28, and 56, and patients were followed until day 112. Blood samples were collected on consecutive days, as indicated in Figure 1 (fasting, before morning drugs and PD 0360324 infusion). Monocytes were immediately stained, analyzed on FACSCanto flow cytometer (BD Biosciences, Immunocytometry Systems), and enumerated as previously described.8 After study closeout, trial unblinding revealed that the female patient received the anti–M-CSF mAbs and the male received placebo.
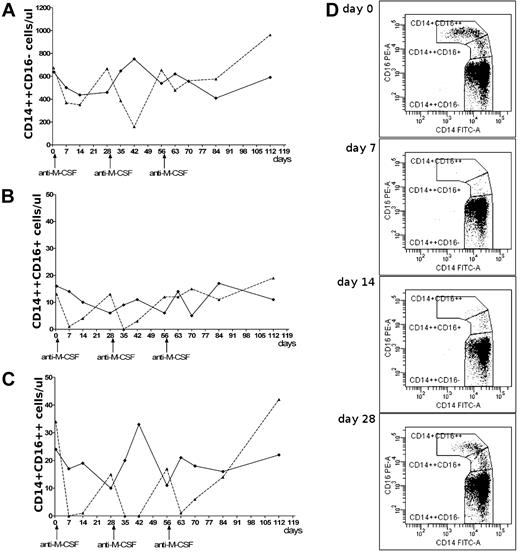
Particular monocyte subpopulations after anti–M-CSF mAb infusions. Changes in the absolute number of circulating classical (A), intermediate (B), and nonclassical (C) monocytes after infusions of anti–M-CSF mAbs (dashed line) or placebo (solid line). (D) The dot plots show changes of the monocyte subpopulations during the first 4 weeks after initial anti–M-CSF mAb infusion in female patient; lack of nonclassical monocytes on day 7, very low number on day 14, and growing number of intermediate monocytes on days 7-28.
Particular monocyte subpopulations after anti–M-CSF mAb infusions. Changes in the absolute number of circulating classical (A), intermediate (B), and nonclassical (C) monocytes after infusions of anti–M-CSF mAbs (dashed line) or placebo (solid line). (D) The dot plots show changes of the monocyte subpopulations during the first 4 weeks after initial anti–M-CSF mAb infusion in female patient; lack of nonclassical monocytes on day 7, very low number on day 14, and growing number of intermediate monocytes on days 7-28.
The number of classical monocytes did not differ between patients. Mean numbers ± SD in antibody-treated patient versus placebo-treated patient were: 530 ± 216 vs 558 ± 104 cells/μL (Figure 1A). However, the nonclassical monocytes disappeared in the female patient after anti–M-CSF mAb infusions (Figure 1C), which was best noticed (for at least 1 week) after 1st and 2nd dosing. Four weeks after each dosing the number of nonclassical monocytes was approaching baseline level and achieved it on day 112. These changes were not observed in the patient on placebo. Similar changes were seen in intermediate monocytes (Figure 1B); however, after infusions their number recovered earlier than that of nonclassical monocytes. This supports previous suggestions that steady-state plasma levels of M-CSF promote differentiation of classical monocytes to intermediate and nonclassical monocytes, and that this process may be inhibited by neutralizing anti–M-CSF mAbs. This is visualized on the dot plots showing dynamic changes of monocyte subpopulations during the first 4 weeks after initial anti–M-CSF mAb infusion in the female patient (Figure 1D). Our data provide, for the first time, direct evidence that M-CSF is essential for differentiation of intermediate and nonclassical monocytes in vivo.
Authorship
Contribution: M.K. was responsible for substudy design, patient enrollment and follow-up, analysis of investigated data, and drafting the manuscript; K.B.S. made cell staining and analyzed cytometric data; S.S. was responsible for clinical study concept and design; T.G. was responsible for substudy concept and critically reviewed the manuscript; and M.S. was responsible for scientific concept of substudy, analysis of investigated data and its interpretation, and drafting the manuscript.
Conflict-of-interest disclosure: M.K. was a primary investigator in Pfizer A6261002 trial and received honoraria from Pfizer. S.S. was an employee of Pfizer. T.G. received honoraria from Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Maciej Siedlar, Department of Clinical Immunology, Polish-American Children Hospital, Jagiellonian University Medical College, Wielicka 265 Str, 30-663 Krakow, Poland; e-mail: misiedla@cyf-kr.edu.pl.