CTLA-4 proteins contribute to the suppressor function of regulatory T cells (Tregs), but the mechanism by which they do so remains incompletely understood. In the present study, we assessed CTLA-4 protein function in both Tregs and conventional (Tconv) CD4+ T cells. We report that CTLA-4 proteins are responsible for all 3 characteristic Treg functions of suppression, TCR hyposignaling, and anergy. However, Treg suppression and anergy only required the external domain of CTLA-4, whereas TCR hyposignaling required its internal domain. Surprisingly, TCR hyposignaling was neither required for Treg suppression nor anergy because costimulatory blockade by the external domain of CTLA-4 was sufficient for both functions. We also report that CTLA-4 proteins were localized in Tregs in submembrane vesicles that rapidly recycled to/from the cell surface, whereas CTLA-4 proteins in naive Tconv cells were retained in Golgi vesicles away from the cell membrane and had no effect on Tconv cell function. However, TCR signaling of Tconv cells released CTLA-4 proteins from Golgi retention and caused activated Tconv cells to acquire suppressor function. Therefore, the results of this study demonstrate the importance of intracellular localization for CTLA-4 protein function and reveal that CTLA-4 protein externalization imparts suppressor function to both regulatory and conventional CD4+ T cells.
Introduction
T cells are selected in the thymus to express TCRs reactive against foreign pathogens but tolerant to self-ligands. However, thymic selection is imperfect, so small numbers of potentially autoreactive T cells invariably escape into the periphery, where their autoreactive potential must be muted by peripheral tolerance mechanisms. Most prominent of these peripheral tolerance mechanisms are T-regulatory cells (Tregs) that suppress the activation of autoreactive T cells in vivo.1–2 Tregs are CD4+CD25+ T cells that express the X-chromosome–linked transcription factor Foxp3.3,,,–7 Foxp3+CD4+CD25+ Tregs possess several unique characteristics that distinguish them from nonregulatory CD4+ T cells. In particular, in addition to possessing the ability to suppress the activation of naive T cells, Tregs themselves have impaired TCR signal transduction and fail to proliferate to antigenic stimulation in the absence of exogenously added IL-2. These 3 functions are characteristic of Tregs and are referred to as suppression, TCR hyposignaling, and anergy.
A protein that is present in Tregs and the expression of which in Tregs is dependent on Foxp3 is CTLA-4.5 Mice with Tregs that lack CTLA-4 protein expression were shown recently to develop lethal autoimmunity, revealing that Treg expression of CTLA-4 was necessary for immune suppression and prevention of in vivo autoimmunity.8–9 A variety of molecular mechanisms for CTLA-4–mediated suppression have been proposed: (1) competition between CTLA-4 and the costimulatory molecule CD28 for binding to their shared APC ligands CD80 and CD8610 ; (2) disruption of CD28 localization in the immunologic synapse11–12 ; (3) inhibition of TCR signaling through recruitment of phosphatases PP2A and SHP-213,–15 ; and (4) shortening of the dwell time between naive T cells and APCs.16 Consequently, CTLA-4 proteins appear to be able to use an impressive variety of different mechanisms to suppress naive T-cell activation; however, little is known about the relationship between the protein structure of CTLA-4 and its various functions. Indeed, it is uncertain whether CTLA-4 proteins only mediate Treg suppression or if they are also responsible for Treg hyposignaling and anergy. Compounding the uncertainty regarding the function of CTLA-4 proteins in Tregs is the fact that CTLA-4 protein expression is not limited to Tregs—CTLA-4 proteins are also expressed in activated conventional (Tconv) T cells.
The present study was undertaken to elucidate CTLA-4 protein function in regulatory T cells and activated Tconv cells. We assessed the role of CTLA-4 proteins in the 3 characteristic Treg functions of suppression, hyposignaling, and anergy, and then examined the CTLA-4 protein domain responsible for each. In addition to assessing CTLA-4 protein function in Tregs, we also assessed CTLA-4 protein in naive and activated Tconv cells. The results of this study significantly enhance our understanding of CTLA-4 protein function in Tregs and identify the functional consequences of CTLA-4 protein expression in activated Tconv cells.
Methods
Animals
Ctla4−/−, CTLA-4TgWT, and CTLA-4TgΔ mice17 were bred in our own animal colony. CTLA-4TgWT and CTLA-4TgΔ were introduced into Ctla4−/− mice so that only transgenic CTLA-4 proteins were expressed. For brevity, we refer to Ctla4−/− mice expressing either of the CTLA-4 transgenes simply as CTLA-4TgWT or CTLA-4TgΔ mice. B6 (CD45.2) mice were purchased from The Jackson Laboratory and B6 (CD45.1) mice were obtained from the Frederick Cancer Research Center (Frederick, MD). All mice were cared for in accordance with National Institutes of Health guidelines.
Generation of Foxp3-transgenic mice
cDNA encoding mouse Foxp3 was synthesized from mouse CD4+CD25+ Tregs, subcloned into pcDNA3 vector (Invitrogen), and then introduced into a human adenosine deaminase–based transgenic expression vector.18 Fragments containing human adenosine deaminase transcriptional control regions and the inserted cDNA were excised from the plasmid backbone and microinjected into fertilized B6 oocytes. Founder mice were identified by Southern blot of DNA obtained from tail tissue. Expression of the transgene was confirmed by PCR amplification of genomic DNA from tail tissue.
Reagents
mAbs with the following specificities were used in this study: CD4 (RM4.4, RM4.5, and GK1.5), CD25 (PC61 and 7D4), CD45.1 (A20), CD45.2 (104), CD152 (UC10-4F10-11), TCR (H57-597), CD44 (1M7), CD62L (MEL-14), and GM130 (35) all obtained from BD Biosciences; and Foxp3 (FJK-16s) obtained from eBiosciences. Stained cells were analyzed on a FACS Vantage SE (BD Biosciences). Where indicated, fluorescence was quantified and expressed as mean fluorescence intensity. CTLA-4Ig was obtained from BD Biosciences.
Construction of radiation BM chimeras
Mixed radiation BM chimeras were generated by reconstituting lethally irradiated (950R) recipient mice with a total of 10 to 15 × 106 T cell–depleted BM cells 6 hours after irradiation. Chimeric mice were analyzed 8 weeks after reconstitution.
Cell preparations
CD4+ lymph node T cells with a purity of > 97% were obtained by Ab-mediated magnetic bead depletion and further fractionated into CD4+CD25+ and CD4+CD25− T-cell populations with a purity > 99% by electronic cell sorting.
In vitro functional assays
For anti-TCR–induced T-cell proliferation, responder T cells (3 to 5 × 104/well) were placed in 96-well round-bottom plates (0.2 mL) together with irradiated T cell–depleted B6 spleen cells (2000R) as accessory cells (APCs) and stimulated with anti-CD3 mAb (1 μg/mL) and/or rIL-2 (200 U/mL) for 72 hours. For in vitro suppression assays, CD4+CD25− responder T cells (3 to 5 × 104/well) were cultured with an equal number of CD4+CD25+ T cells, APCs, and anti-CD3 mAb (1 μg/mL) for 72 hours. Where indicated, cultures were pulsed with [3H]-thymidine 8 hours before harvest. Alternatively, CFSE-labeled CD4+CD25− responder T cells were cocultured with CD4+CD25+ and APCs (both of which expressed a different CD45 allele from the Tconv cells) and stimulated with anti-CD3 (1 μg/mL) for 72 hours. At the end of culture, CFSE fluorescence of the responder T cells was determined. For preactivated T cell–induced suppression, purified CD4+CD25− T cells were activated with plate-bound anti-TCR (2 μg/mL), anti-CD28 (5 μg/mL), and IL-2 (200 U/mL) overnight, cocultured with CFSE-labeled responder T cells and APCs, and then stimulated with anti-CD3 (1 μg/mL) for 72 hours.
Intracellular staining
To detect intracellular CTLA-4 and Foxp3 levels, purified lymph node CD4+ T cells were surfaced stained with anti-CD4 and anti-CD25, fixed, and then stained for CTLA-4 and Foxp3.
Calcium mobilization
Cells were loaded with the calcium-sensitive dye Indo-1 at 37°C and then coated at 4°C with PE-labeled anti-CD25 and biotinylated mAbs specific for TCRβ and CD4 (GK1.5). Coated cells were kept at 4°C until 2 minutes before stimulation, when cells were warmed and analyzed by flow cytometry. Ab cross-linking was induced with avidin (4 μg/mL), and data acquisition was recorded for 5 minutes. Intracellular calcium concentrations were determined by the ratio of Indo-1 fluorescence at 405 versus 510 nm.
T-cell reconstitution and histology
RAG-2−/− mice were injected intravenously with 4 × 105 purified CD4+CD45RBhigh T cells either alone or in combination with 4 × 105 CD4+CD25+ T cells from the indicated sources. After T-cell transfer, mice were weighed weekly and monitored for clinical signs of diarrhea. Colons from experimental mice were fixed in 10% buffered formalin, embedded in paraffin, sectioned, and stained with H&E.
Immunofluorescence microscopy
To detect colocalization of TCR and CTLA-4, purified T cells were first surface stained with Alexa Fluor 647–labeled anti-TCR and then adhered onto poly-D-lysine–coated coverslips for 10-20 minutes at room temperature. Cells were fixed with 4% paraformaldehyde in PBS, permeabilized with 1% NP40/0.01% saponin, and then stained with PE-labeled anti–CTLA-4. To detect colocalization of GM130, fixed cells were stained with Alexa Fluor 647–labeled GM130 and PE-labeled anti–CTLA-4 after fixation/permeabilization. Samples were imaged using a 40× C-Apochromat (numerical aperture 1.2) water immersion lens coupled to a Zeiss LSM510 META confocal microscope (Carl Zeiss MicroImaging). The 3D models of staining were reconstituted using Imaris Version 5.5 software (Bitplane).
Results
Structure/function analysis of CTLA-4 proteins in Treg function
To understand the contribution of CTLA-4 proteins to Treg function, we reconstituted Ctla4−/− mice with transgenes encoding CTLA-4 proteins that were either full-length or truncated (lacking the internal domain of CTLA-4). We refer to these transgenes as CTLA-4TgWT and CTLA-4TgΔ, respectively. Both CTLA-4 transgenes were introduced into Ctla4−/− mice so that Tregs from transgenic mice expressed only transgenic CTLA-4 proteins and Tregs from unreconstituted Ctla4−/− mice expressed no CTLA-4 proteins at all. However, mice with Tregs lacking full-length CTLA-4 molecules (ie, CTLA-4TgΔ and Ctla4−/− mice) were at risk for developing in vivo autoimmunity, which we feared might indirectly obscure Treg function. To avoid this concern, we always obtained Tregs of CTLA-4TgΔ and Ctla4−/− genetic origins from mixed BM chimeras constructed with transgenic and B6 donors (Table 1). Such mixed BM chimeras contained Foxp3+ Tregs of both donor origins and did not develop autoimmunity (supplemental Figure 1A [available on the Blood Web site; see the Supplemental Materials link at the top of the online article], and Table 1). Indeed, B6+Ctla4−/−→B6 chimeric mice remained healthy and contained conventional CD4+ T cells that displayed a naive (CD25−CD62LhiCD44lo) phenotype, whereas intact Ctla4−/− mice suffered from a lymphoproliferative disease and contained CD4+ T cells that displayed an activated (CD25+CD62LloCD44hi) phenotype (supplemental Figure 1B). We compared Tregs of CTLA-4TgWT, CTLA-4TgΔ, and Ctla4−/− genetic origins for 3 characteristic Treg functions: suppression, TCR hyposignaling, and anergy.
Mouse origin of lymph node T cells used in the current study
| Mouse origin . | Genetic origin . | Frequency of Foxp3+CD4+ T cells . |
|---|---|---|
| B6+Ctla4−/−→B6 | B6 | 18% ± 6.2% |
| Ctla4−/− | 8% ± 3.1% | |
| CTLA-4TgWT | CTLA-4TgWT | 7% ± 1.8% |
| B6+CTLA-4TgΔ →B6 | B6 | 16% ± 4.6% |
| CTLA-4TgΔ | 7% ± 1.1% |
| Mouse origin . | Genetic origin . | Frequency of Foxp3+CD4+ T cells . |
|---|---|---|
| B6+Ctla4−/−→B6 | B6 | 18% ± 6.2% |
| Ctla4−/− | 8% ± 3.1% | |
| CTLA-4TgWT | CTLA-4TgWT | 7% ± 1.8% |
| B6+CTLA-4TgΔ →B6 | B6 | 16% ± 4.6% |
| CTLA-4TgΔ | 7% ± 1.1% |
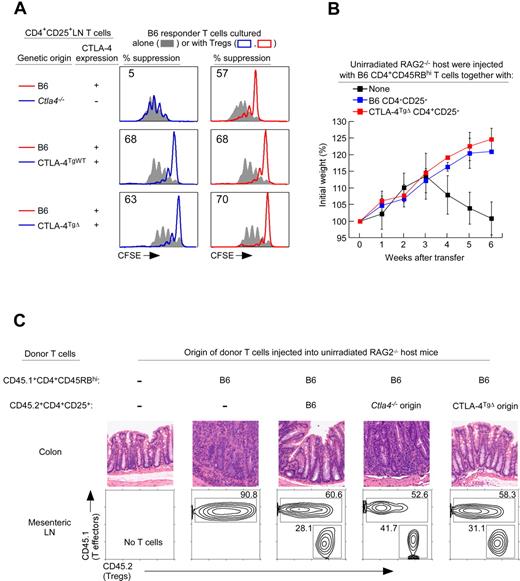
To assess the contribution of CTLA-4 proteins to Treg suppression, we examined their ability to suppress in vitro proliferative responses of Tconv cells stimulated by anti-CD3 mAb and APCs (Figure 1A). As measured by CFSE dye dilution, Tconv cell-proliferative responses were suppressed by CTLA-4–replete B6 Tregs, but not by CTLA-4–deficient Ctla4−/− Tregs, revealing that Treg suppression required CTLA-4 proteins (Figure 1A top panels). Moreover, Tconv cell-proliferative responses were suppressed by Tregs expressing either full-length or truncated CTLA-4 proteins from CTLA-4TgWT and CTLA-4TgΔ mice (Figure 1A left panels), demonstrating that the external protein domain of CTLA-4 was sufficient for Treg suppression of in vitro proliferative responses by Tconv cells.
CTLA-4 external domain confers Treg suppression. (A) Treg suppression as assessed by CFSE dye dilution. CFSE-labeled CD4+CD25− lymph node Tconv cells from normal B6 mice were either cultured alone (filled curves) or cultured together with purified CD4+CD25+ Tregs of different mice origin and stimulated to proliferate by anti-CD3 (1 μg/mL) and APCs bearing CD80/86–costimulatory ligands. Tregs from Ctla4−/− and CTLA-4TgΔ origins were purified from mixed BM chimeras. Proliferation of Tconv cells was assessed by CFSE dye dilution. The percentage of Tconv cells that failed to divide at least once in cocultures is shown. Data are representative of 4 independent experiments. (B) Five-week-old RAG-2–deficient mice were injected with 4 × 105 CD4+CD45RBhigh (CD45.1+) T cells alone or together with 4 × 105 purified CD4+CD25+ Treg cells (CD45.2) of either B6 or CTLA-4TgΔ donor origin obtained from B6+CTLA-4TgΔ→B6 mice. The percentage change from initial body weight of the recipients was monitored over time. The initial weight gain was because of the young age of the recipient mice. Each data point represents 3 or 4 RAG2−/− recipient mice. (C) RAG-2–deficient mice were injected with 4 × 105 CD4+CD45RBhigh (CD45.1+) T cells alone or with 4 × 105 purified CD4+CD25+ (CD45.2) Tregs of either Ctla4−/− or CTLA-4TgΔ origin obtained from mixed BM chimeras. Tregs of B6 origin were used as a positive control. Five weeks later, mice were killed and their colons stained with H&E. Images are representative of 4 mice from each group. Staining of mesenteric lymph nodes for the relative proportions of CD4+CD45RBhigh effectors and CD4+CD25+ Tregs is shown.
CTLA-4 external domain confers Treg suppression. (A) Treg suppression as assessed by CFSE dye dilution. CFSE-labeled CD4+CD25− lymph node Tconv cells from normal B6 mice were either cultured alone (filled curves) or cultured together with purified CD4+CD25+ Tregs of different mice origin and stimulated to proliferate by anti-CD3 (1 μg/mL) and APCs bearing CD80/86–costimulatory ligands. Tregs from Ctla4−/− and CTLA-4TgΔ origins were purified from mixed BM chimeras. Proliferation of Tconv cells was assessed by CFSE dye dilution. The percentage of Tconv cells that failed to divide at least once in cocultures is shown. Data are representative of 4 independent experiments. (B) Five-week-old RAG-2–deficient mice were injected with 4 × 105 CD4+CD45RBhigh (CD45.1+) T cells alone or together with 4 × 105 purified CD4+CD25+ Treg cells (CD45.2) of either B6 or CTLA-4TgΔ donor origin obtained from B6+CTLA-4TgΔ→B6 mice. The percentage change from initial body weight of the recipients was monitored over time. The initial weight gain was because of the young age of the recipient mice. Each data point represents 3 or 4 RAG2−/− recipient mice. (C) RAG-2–deficient mice were injected with 4 × 105 CD4+CD45RBhigh (CD45.1+) T cells alone or with 4 × 105 purified CD4+CD25+ (CD45.2) Tregs of either Ctla4−/− or CTLA-4TgΔ origin obtained from mixed BM chimeras. Tregs of B6 origin were used as a positive control. Five weeks later, mice were killed and their colons stained with H&E. Images are representative of 4 mice from each group. Staining of mesenteric lymph nodes for the relative proportions of CD4+CD45RBhigh effectors and CD4+CD25+ Tregs is shown.
To determine whether the external domain of CTLA-4 was also sufficient for Tregs to suppress in vivo responses, we assessed the ability of different Tregs to suppress the in vivo induction of inflammatory bowel disease (IBD). Transfer of CD4+CD45RBhi T-effector cells into unirradiated RAG-2−/− mice induced severe diarrhea that resulted in weight loss (Figure 1B), death (Table 2), and histologic evidence of IBD with lymphocytic infiltration and crypt destruction (Figure 1C). Cotransfer of Tregs of B6 origin suppressed the in vivo induction of IBD, but cotransfer of CTLA-4–deficient Tregs of Ctla4−/− origin failed to suppress IBD (Figure 1B-C and Table 2), even though both Treg populations successfully engrafted and populated the mesenteric lymph nodes of host mice (Figure 1C bottom panels). The in vivo suppressive function of Ctla4−/− Tregs was fully restored by CTLA-4TgΔ proteins, because CTLA-4TgΔ Tregs suppressed the in vivo induction of IBD (Figure 1B-C). Therefore, by both in vitro and in vivo assessments, the external protein domain of CTLA-4 was sufficient for Treg suppression of Tconv cell responses.
Treg expression of CTLA-4 is required for in vivo suppression of autoreactive T cells
| Unirradiated host . | CD45RBhi donor T cells . | Donor CD4+CD25+ Tregs . | Survival at 18 wk . | |
|---|---|---|---|---|
| Mouse origin . | Genetic origin . | |||
| RAG-2−/− | B6 | None | None | 0/4 |
| RAG-2−/− | B6 | B6+Ctla4−/−→B6 | B6CD45.1 | 4/4 |
| RAG-2−/− | B6 | B6+Ctla4−/−→B6 | Ctla4−/−CD45.2 | 0/4 |
| Unirradiated host . | CD45RBhi donor T cells . | Donor CD4+CD25+ Tregs . | Survival at 18 wk . | |
|---|---|---|---|---|
| Mouse origin . | Genetic origin . | |||
| RAG-2−/− | B6 | None | None | 0/4 |
| RAG-2−/− | B6 | B6+Ctla4−/−→B6 | B6CD45.1 | 4/4 |
| RAG-2−/− | B6 | B6+Ctla4−/−→B6 | Ctla4−/−CD45.2 | 0/4 |
RAG-2−/− mice were injected with CD4+CD45RBhigh (B6) cells either alone or together with sorted CD4+CD25+ donor cells of B6 or Ctla4−/−origin that were isolated from B6+Ctla4−/−→B6 mixed chimeras. Mice were observed daily and their survival at 18 weeks is noted.
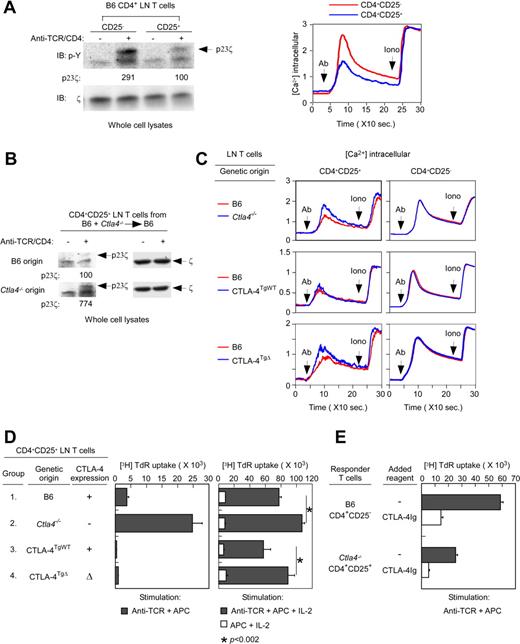
Having determined that the external domain of CTLA-4 was sufficient for Treg suppressor function, we then assessed the effect of CTLA-4 proteins on TCR signaling. CD4+CD25+ Tregs are signaled poorly by TCR stimulation compared with CD4+CD25− Tconv cells,19 but the reason for this is not known. Therefore, we measured TCR signaling in CD4+CD25− Tconv and CD4+CD25+ Tregs by both tyrosine phosphorylation and calcium mobilization. As revealed by p23 phospho-ζ and calcium mobilization, TCR signaling was substantially diminished in Tregs compared with Tconv cells (Figure 2A), confirming that TCR signaling was impaired in Tregs. As measured by both ζ phosphorylation and calcium mobilization, TCR signaling was greater in CTLA-4–deficient Ctla4−/− Tregs than in CTLA-4–replete B6 Tregs (Figure 2B and C left top panel), revealing that CTLA-4 proteins were in fact responsible for impaired TCR signaling.
Effect of CTLA-4 on TCR signaling and anergy. (A) Impairment of TCR signaling in Tregs from normal B6 mice. In the left panel, whole-cell lysates from CD4+CD25− and CD4+CD25+ B6 lymph node T cells that were either unstimulated or stimulated by anti–TCR/CD4 cross-linking (in the absence of APCs) were blotted for p23 phospho-ζ (p23ζ) with a phospho-tyrosine (p-Y)–specific Ab. The p23ζ band intensity from anti-TCR/CD4–stimulated CD4+CD25+ T cells was set to 100. As an intrinsic loading control, membranes were also blotted for total ζ with a ζ-specific Ab. Data are representative of 3 independent experiments. In the right panel, calcium flux was induced in Indo-1–loaded CD4+CD25− and CD4+CD25+ B6 lymph node T cells by avidin-induced cross-linking of biotinylated anti-TCR/CD4 mAbs. Data are representative of 6 independent experiments. (B) TCR-induced ITAM phosphorylation in Tregs of different donor origin. Whole-cell lysates from CD4+CD25+ lymph node T cells of different origin from B6+Ctla4−/−→B6 mice that were either unstimulated or stimulated by anti–TCR/CD4 cross-linking (in the absence of APCs) were blotted for p23ζ. The p23ζ band intensity from anti–TCR/CD4–stimulated CD4+CD25+ T cells of B6 origin was set to 100. As an intrinsic loading control, membranes were also blotted for total ζ. Data are representative of 2 independent experiments from 20 mixed BM chimeras. (C) The CTLA-4 internal domain is responsible for TCR hyposignaling. TCR-induced calcium mobilization in peripheral T cells was induced in Indo-1–loaded CD4+CD25− and CD4+CD25+ lymph node T cells by avidin-induced cross-linking of biotinylated anti–TCR/CD4 mAbs or by ionomycin (in the absence of APC). In mixed-radiation BM chimeras, B6 donor origin T cells were identified as CD45.2− T cells, whereas Ctla4−/−, CTLA-4TgWT or CTLA-4TgΔ origin T cells were identified as CD45.2+. Data for each experimental group are representative of 4 independent experiments. (D) The CTLA-4 external domain induces Treg anergy. Tregs from the indicated populations of CD4+CD25+ lymph node T cells were sorted and stimulated by anti-CD3 (1 μg/mL) with APC (left panel) or by APC with rIL-2 (200 U/mL) with or without anti-CD3 (right panel). Tregs from Ctla4−/− and CTLA-4TgΔ origins were purified from mixed BM chimeras. Proliferation was measured by 3H-thymidine incorporation and mean cpm ± SD of triplicate wells are shown. Data are representative of 3 independent experiments. (E) B6 lymph node CD4+CD25− Tconv cells and Ctla4−/− origin Tregs from B6+Ctla4−/−→B6 mice were stimulated by anti-CD3 (1 μg/mL) and APCs in the presence or absence of CTLA-4Ig (10 μg/mL). Proliferation was measured by 3H-thymidine incorporation and mean cpm ± SD of triplicate wells are shown. Data are representative of 2 independent experiments.
Effect of CTLA-4 on TCR signaling and anergy. (A) Impairment of TCR signaling in Tregs from normal B6 mice. In the left panel, whole-cell lysates from CD4+CD25− and CD4+CD25+ B6 lymph node T cells that were either unstimulated or stimulated by anti–TCR/CD4 cross-linking (in the absence of APCs) were blotted for p23 phospho-ζ (p23ζ) with a phospho-tyrosine (p-Y)–specific Ab. The p23ζ band intensity from anti-TCR/CD4–stimulated CD4+CD25+ T cells was set to 100. As an intrinsic loading control, membranes were also blotted for total ζ with a ζ-specific Ab. Data are representative of 3 independent experiments. In the right panel, calcium flux was induced in Indo-1–loaded CD4+CD25− and CD4+CD25+ B6 lymph node T cells by avidin-induced cross-linking of biotinylated anti-TCR/CD4 mAbs. Data are representative of 6 independent experiments. (B) TCR-induced ITAM phosphorylation in Tregs of different donor origin. Whole-cell lysates from CD4+CD25+ lymph node T cells of different origin from B6+Ctla4−/−→B6 mice that were either unstimulated or stimulated by anti–TCR/CD4 cross-linking (in the absence of APCs) were blotted for p23ζ. The p23ζ band intensity from anti–TCR/CD4–stimulated CD4+CD25+ T cells of B6 origin was set to 100. As an intrinsic loading control, membranes were also blotted for total ζ. Data are representative of 2 independent experiments from 20 mixed BM chimeras. (C) The CTLA-4 internal domain is responsible for TCR hyposignaling. TCR-induced calcium mobilization in peripheral T cells was induced in Indo-1–loaded CD4+CD25− and CD4+CD25+ lymph node T cells by avidin-induced cross-linking of biotinylated anti–TCR/CD4 mAbs or by ionomycin (in the absence of APC). In mixed-radiation BM chimeras, B6 donor origin T cells were identified as CD45.2− T cells, whereas Ctla4−/−, CTLA-4TgWT or CTLA-4TgΔ origin T cells were identified as CD45.2+. Data for each experimental group are representative of 4 independent experiments. (D) The CTLA-4 external domain induces Treg anergy. Tregs from the indicated populations of CD4+CD25+ lymph node T cells were sorted and stimulated by anti-CD3 (1 μg/mL) with APC (left panel) or by APC with rIL-2 (200 U/mL) with or without anti-CD3 (right panel). Tregs from Ctla4−/− and CTLA-4TgΔ origins were purified from mixed BM chimeras. Proliferation was measured by 3H-thymidine incorporation and mean cpm ± SD of triplicate wells are shown. Data are representative of 3 independent experiments. (E) B6 lymph node CD4+CD25− Tconv cells and Ctla4−/− origin Tregs from B6+Ctla4−/−→B6 mice were stimulated by anti-CD3 (1 μg/mL) and APCs in the presence or absence of CTLA-4Ig (10 μg/mL). Proliferation was measured by 3H-thymidine incorporation and mean cpm ± SD of triplicate wells are shown. Data are representative of 2 independent experiments.
To determine which CTLA-4 domains were responsible for TCR hyposignaling, we assessed TCR signaling in Tregs expressing different CTLA-4 protein domains. We found that TCR signaling in Ctla4−/− Tregs was reduced to B6 levels by full-length CTLA-4TgWT proteins (Figure 2C left middle panel), but was not at all reduced by truncated CTLA-4TgΔ proteins (Figure 2C left bottom panel). Therefore, these results demonstrated that TCR hyposignaling required CTLA-4 proteins containing the internal domain of CTLA-4. Such impairment by the internal domain of CTLA-4 significantly reduced TCR signaling of costimulation-independent proliferation in the presence of exogenous IL-2 (Figure 2D right, compare groups 1 and 2 with groups 3 and 4).
Finally, we assessed the role of CTLA-4 proteins in mediating Treg anergy, which refers to the inability of Tregs to proliferate in response to anti–TCR/APC stimulation in the absence of exogenously added IL-2. We found that CTLA-4–replete B6 Tregs failed to proliferate to anti–TCR/APC stimulation without exogenous IL-2, whereas CTLA-4–deficient Ctla4−/− Tregs proliferated vigorously (Figure 2D left, groups 1 and 2), demonstrating that CTLA-4 proteins were responsible for Treg anergy. Moreover, we found that the responsiveness of Ctla4−/− Tregs to anti-TCR/APC stimulation was dampened by both full-length CTLA-4TgWT and truncated CTLA-4TgΔ proteins (Figure 2D left, groups 3 and 4), revealing that Treg anergy resembled Treg suppression in only requiring the external domain of CTLA-4 which binds CD80/86–costimulatory ligands on APCs with high affinity.20
Because we had considered Treg anergy to be a manifestation of TCR hyposignaling, we were surprised that these 2 Treg functions (anergy and hyposignaling) required different CTLA-4 domains. Indeed, the external domain of CTLA-4 was itself sufficient for Treg anergy and Treg suppression, but the internal domain of CTLA-4 was required for TCR hyposignaling. To understand why the external domain of CTLA-4 was sufficient for both Treg suppression and anergy, we considered that these Treg functions might involve costimulatory blockade induced by high-affinity binding by the external domain of CTLA-4 to CD80 and CD86 costimulatory ligands on APCs. If this were the case, then CTLA-4Ig, a soluble form of the external domain of CTLA-4, should mimic both Treg suppression and Treg anergy. Indeed, we found that soluble CTLA-4Ig did block CD4+CD25− Tconv cell responses to anti-CD3/APC stimulation (Figure 2E and supplemental Figure 2), mimicking Treg suppression, and that soluble CTLA-4Ig also blocked CD4+CD25+ Treg proliferative responses induced by anti-CD3/APC stimulation (Figure 2E and supplemental Figure 2), mimicking Treg anergy. Based on these results, we conclude that Treg anergy reflects CTLA-4–induced “auto-suppression” and that costimulatory blockade induced by the external domain of CTLA-4 is sufficient for both Treg suppression and Treg anergy.
We conclude that CTLA-4 proteins are required for all 3 characteristic Treg functions of suppression, TCR hyposignaling, and anergy. However, Treg suppression and Treg anergy only require the external domain of CTLA-4, whereas TCR hyposignaling requires the internal domain of CTLA-4.
TCR-signaling promotes CTLA-4 externalization
Because CTLA-4 transgene expression was driven by the lck promotor/enhancer, transgenic CTLA-4 proteins were expressed in both CD4+CD25+ Tregs and CD4+CD25− Tconv cells. In fact, CD4+CD25− Tconv cells from CTLA-4TgWT mice actually contained greater amounts of intracellular CTLA-4 than CD4+CD25+ Tregs from nontransgenic B6 mice (supplemental Figure 3). Nevertheless, CTLA-4 proteins had no apparent effect on naive Tconv cells, because CD4+CD25− Tconv cells from CTLA-4TgWT mice were neither hyporesponsive (Figure 2C middle right panel) nor suppressive (Figure 3A). To understand this finding, we sought to visualize directly the intracellular location of transgenic CTLA-4 proteins in Tconv and Treg cells.
External expression of CTLA-4 is crucial for CTLA-4–mediated suppression. (A) Resting CTLA-4TgWT CD4+CD25− lymph node T cells are not suppressive. CFSE-labeled CD4+CD25− Tconv cells from lymph nodes of normal B6 (CD45.1) mice were either cultured alone (filled curves) or together with purified CD4+CD25− T cells from B6 or CTLA-4TgWT (ie, Ctla4−/−CTLA-4TgWT) mice (open curves) and stimulated to proliferate by anti-CD3 (1 μg/mL) and APCs. Proliferation of CD45.1+ Tconv cells was assessed by CFSE dye dilution. The percentage of Tconv cells that failed to divide at least once in cocultures is shown. Data are representative of 3 independent experiments. (B-C) Intracellular localization of CTLA-4 proteins in CD4+CD25+ Tregs and CD4+CD25− Tconv cells. Resting T cells and T cells preactivated for 2 hours by anti-TCR were stained for TCR, CTLA-4, and GM130. In panel B, T cells were stained for surface TCR (shown in red) followed by fixation and staining for intracellular CTLA-4 (shown in green) and were then visualized by confocal microscopy (see also supplemental Figure 3 and supplemental Videos 1-2). In panel C, T cells were fixed and stained for intracellular CTLA-4 (shown in green) and GM130 (shown in red; see also supplemental Figure 3 and supplemental Videos 3-4) and then visualized by confocal microscopy. (D) Assessment of CTLA-4 externalization in Tregs and Tconv cells. To compare the rate of CTLA-4 recycling and externalization in Tregs and CTLA-4TgWTTconv cells, resting CD4+CD25+ Tregs of B6 origin and resting CD4+CD25− Tconv cells of either CTLA-4TgWT or Ctla4−/− origin were cultured (without APCs) at 37°C for 60 minutes with PE-conjugated anti–CTLA-4 mAb in plates that had been coated either with medium alone or with anti-TCR/CD28 mAbs. The PE-conjugated anti–CTLA-4 mAb was added to capture CTLA-4 proteins cycling to the surface and was detected as surface PE fluorescence. The PE fluorescence acquired in 60 minutes by the indicated T-cell populations during culture in medium alone (blue line) or during culture on plate-bound anti-TCR/CD28 mAbs (red line) is displayed. Data are representative of 2 independent experiments. (E) Preactivation makes CD4+CD25− T cells from CTLA-4TgWT mice suppressive. CFSE-labeled CD4+CD25− lymph node Tconv cells from normal B6 mice were cultured alone (filled curves) or with the indicated T-cell populations (open curves) and stimulated to proliferate by anti-CD3 (1 μg/mL) and APCs. Where indicated, preactivated T cells were stimulated overnight with anti-TCR/CD28+IL-2 before addition to the stimulation cultures. The percentage of Tconv cells that failed to divide at least once in cocultures is shown. Data are representative of 3 independent experiments.
External expression of CTLA-4 is crucial for CTLA-4–mediated suppression. (A) Resting CTLA-4TgWT CD4+CD25− lymph node T cells are not suppressive. CFSE-labeled CD4+CD25− Tconv cells from lymph nodes of normal B6 (CD45.1) mice were either cultured alone (filled curves) or together with purified CD4+CD25− T cells from B6 or CTLA-4TgWT (ie, Ctla4−/−CTLA-4TgWT) mice (open curves) and stimulated to proliferate by anti-CD3 (1 μg/mL) and APCs. Proliferation of CD45.1+ Tconv cells was assessed by CFSE dye dilution. The percentage of Tconv cells that failed to divide at least once in cocultures is shown. Data are representative of 3 independent experiments. (B-C) Intracellular localization of CTLA-4 proteins in CD4+CD25+ Tregs and CD4+CD25− Tconv cells. Resting T cells and T cells preactivated for 2 hours by anti-TCR were stained for TCR, CTLA-4, and GM130. In panel B, T cells were stained for surface TCR (shown in red) followed by fixation and staining for intracellular CTLA-4 (shown in green) and were then visualized by confocal microscopy (see also supplemental Figure 3 and supplemental Videos 1-2). In panel C, T cells were fixed and stained for intracellular CTLA-4 (shown in green) and GM130 (shown in red; see also supplemental Figure 3 and supplemental Videos 3-4) and then visualized by confocal microscopy. (D) Assessment of CTLA-4 externalization in Tregs and Tconv cells. To compare the rate of CTLA-4 recycling and externalization in Tregs and CTLA-4TgWTTconv cells, resting CD4+CD25+ Tregs of B6 origin and resting CD4+CD25− Tconv cells of either CTLA-4TgWT or Ctla4−/− origin were cultured (without APCs) at 37°C for 60 minutes with PE-conjugated anti–CTLA-4 mAb in plates that had been coated either with medium alone or with anti-TCR/CD28 mAbs. The PE-conjugated anti–CTLA-4 mAb was added to capture CTLA-4 proteins cycling to the surface and was detected as surface PE fluorescence. The PE fluorescence acquired in 60 minutes by the indicated T-cell populations during culture in medium alone (blue line) or during culture on plate-bound anti-TCR/CD28 mAbs (red line) is displayed. Data are representative of 2 independent experiments. (E) Preactivation makes CD4+CD25− T cells from CTLA-4TgWT mice suppressive. CFSE-labeled CD4+CD25− lymph node Tconv cells from normal B6 mice were cultured alone (filled curves) or with the indicated T-cell populations (open curves) and stimulated to proliferate by anti-CD3 (1 μg/mL) and APCs. Where indicated, preactivated T cells were stimulated overnight with anti-TCR/CD28+IL-2 before addition to the stimulation cultures. The percentage of Tconv cells that failed to divide at least once in cocultures is shown. Data are representative of 3 independent experiments.
Using confocal microscopy, we examined CD4+CD25− Tconv cells and CD4+CD25+ Tregs from CTLA-4TgWT mice and found that CTLA-4–transgenic proteins in CD4+CD25+ Tregs were dispersed in submembrane vesicles near the plasma membrane, which we identified by anti-TCR staining, whereas the same transgenic CTLA-4 proteins in CD4+CD25− Tconv cells were not dispersed near the plasma membrane (Figure 3B and supplemental Videos 1-2). Instead, transgenic CTLA-4 proteins in CD4+CD25− Tconv cells were localized in perinuclear Golgi vesicles, which we identified by the Golgi-resident protein GM130 (Figure 3C and supplemental Videos 3-4). Therefore, transgenic CTLA-4 proteins were in different intracellular locations in Tregs and Tconv cells. We considered that Golgi retention of transgenic CTLA-4 proteins in Tconv cells might have been due either to the lack of Foxp3 or to the lack of stimulatory high-affinity TCR interactions with in vivo self-antigens. To first assess the possibility that Foxp3 expression affected intracellular CTLA-4 protein localization, we constructed a Foxp3 transgene (Foxp3Tg) and introduced it into CTLA-4TgWT mice (Figure 3B and supplemental Figure 4). However, expression of transgenic Foxp3 did not affect Golgi retention of transgenic CTLA-4 proteins in CD4+CD25− Tconv cells (Figure 3B). We then examined the possibility that Golgi retention of CTLA-4 might be reversed by preactivating Tconv cells with anti-TCR mAb. Interestingly, anti-TCR stimulation released transgenic CTLA-4 proteins from the Golgi of Tconv cells so that CTLA-4 proteins were now dispersed in submembrane vesicles near the plasma membrane (Figure 3C). Therefore, direct visualization by confocal microscopy revealed that TCR-mediated preactivation of Tconv cells diminished Golgi retention of CTLA-4 proteins substantially.
Consistent with their expression in submembrane vesicles near the plasma membrane, CTLA-4 proteins in Tregs are not stably expressed on the cell surface, but are rapidly cycling between the cell surface and the cell interior.21 The rapid recycling of CTLA-4 can be revealed by the addition of PE-conjugated anti–CTLA-4 mAb to cultures of Tregs that bind and retain CTLA-4 proteins that have cycled to the cell surface (Figure 3D). In fact, unstimulated Tregs progressively accumulated anti–CTLA-4 surface fluorescence (Figure 3D left panel) specifically, because anti–CTLA-4 surface fluorescence did not accumulate on CTLA-4–deficient Ctla4−/− T cells (Figure 3D right panel). We then used this assay to determine whether CTLA-4 proteins in transgenic Tconv cells similarly recycled between the cell surface and the cell interior. However, unlike the extensive accumulation of anti–CTLA-4 surface fluorescence on unstimulated Tregs, we observed only a small amount of CTLA-4 surface fluorescence on unstimulated Tconv cells (Figure 3D compare left and middle panels). However, anti-TCR stimulation substantially increased CTLA-4 externalization by Tconv cells to the point that CTLA-4 externalization was as extensive on preactivated Tconv cells as on Tregs (Figure 3D compare left and middle panels). We conclude that TCR signaling induces CTLA-4 externalization in Tconv cells.
Preactivation induces CTLA-4–replete Tconv cells to become suppressive
Because TCR signaling promoted CTLA-4 externalization and because the external domain of CTLA-4 was sufficient to mediate suppression, we wondered if CTLA-4–replete Tconv cells might acquire suppressor function when preactivated by anti-TCR stimulation. To assess this possibility, we examined the effect of CD4+CD25− Tconv cells from CTLA-4TgWT mice on the proliferative response of B6 Tconv cells to anti–CD3/APC stimulation (Figure 3E). Without preactivation, CTLA-4TgWT Tconv cells did not affect the proliferative response of B6 Tconv cells, and this was not changed by expression of a Foxp3 transgene (Figure 3E). Remarkably, however, preactivated CTLA-4TgWT Tconv cells did suppress proliferative responses by B6 Tconv cells, and the preactivated Tconv cells were suppressive regardless of whether they expressed a Foxp3 transgene (Figure 3E bottom panels). Therefore, TCR signaled preactivation of CTLA-4–transgenic Tconv cells induced both CTLA-4 externalization and acquisition of suppressor function.
Preactivation induces normal Tconv cells to express CTLA-4 and exert suppressor function
TCR preactivation is known to induce CTLA-4 protein expression in normal (ie, nontransgenic) Tconv cells.10,22,–24 Because our current findings revealed that TCR preactivation induces CTLA-4 externalization in Tconv cells and that the external domain of CTLA-4 is sufficient for suppression, we considered the possibility that TCR preactivation might also induce normal Tconv cells to acquire suppressor function. To test this surprising prediction, we preactivated CD4+CD25− Tconv cells from normal B6 mice for 24 hours with anti-TCR/CD28 and IL-2 in vitro and confirmed that they now expressed CTLA-4 proteins in submembrane vesicles proximal to the plasma membrane (Figure 4A). Remarkably, preactivated CD4+CD25− B6 Tconv cells did suppress in vitro proliferative responses of CFSE-labeled naive B6 Tconv cells to anti-CD3/APC stimulation potently (Figure 4B). In fact, preactivated CD4+CD25− B6 Tconv cells were as suppressive as conventional CD4+CD25+ B6 Tregs (Figure 4B). Therefore, TCR preactivation of normal Tconv cells induces CTLA-4 expression and externalization and potent suppressor function.
CTLA-4 externalization confers suppressor function to preactivated Tconv cells. (A) CTLA-4 expression in preactivated Tconv cells from B6 mice. CTLA-4 expression in resting B6 CD4+CD25+, resting B6 CD4+CD25−, and preactivated B6 CD4+CD25− cells are displayed (shown in green). Preactivated B6 CD4+CD25− T cells were purified B6 CD4+CD25− T cells that had been stimulated overnight by plate-bound anti–TCR/CD28+IL-2 (in the absence of APCs). (B) Preactivation makes Tconv cells from B6 mice suppressive. CFSE-labeled CD4+CD25− lymph node Tconv cells from normal B6 mice (CD45.1+) were either cultured alone (filled curves) or with resting B6 CD4+CD25+ Tregs and resting or preactivated B6 CD4+CD25− lymph node T cells (open curves), and were stimulated to proliferate by anti-CD3 (1 μg/mL) and APCs. Preactivated B6 CD4+CD25− T cells were obtained as in panel A. The percentage of Tconv cells that failed to divide at least once in cocultures is shown. (C) Suppression by preactivated Tconv cells requires CTLA-4 externalization and expression. Preactivated or resting CD4+CD25− lymph node T cells of B6, Ctla4−/−, CTLA-4TgWT, or CTLA-4TgΔ origin were cocultured with CFSE-labeled B6 CD4+CD25− lymph node Tconv cells and stimulated to proliferate by anti-CD3 (1 μg/mL) and APCs. Lymph node T cells of Ctla4−/− and CTLA-4TgΔ origin were purified from mixed BM chimeras. In the left panel, Tconv cell proliferation was assessed by CFSE dilution. The percentage of suppression = 100*[(% of Tconv cells cultured alone that had divided at least once) − (% of Tconv cells from Treg cocultures that had divided at least once)] / (% of Tconv cells alone that had divided at least once). Means ± SE of 3 independent experiments and statistically significant differences as determined by the 2-tailed Student t test are indicated. In the right panel, CD25 surface staining on preactivated or resting CD4+CD25− T cells was quantified into mean fluorescence intensity. Data are representative of 3 independent experiments.
CTLA-4 externalization confers suppressor function to preactivated Tconv cells. (A) CTLA-4 expression in preactivated Tconv cells from B6 mice. CTLA-4 expression in resting B6 CD4+CD25+, resting B6 CD4+CD25−, and preactivated B6 CD4+CD25− cells are displayed (shown in green). Preactivated B6 CD4+CD25− T cells were purified B6 CD4+CD25− T cells that had been stimulated overnight by plate-bound anti–TCR/CD28+IL-2 (in the absence of APCs). (B) Preactivation makes Tconv cells from B6 mice suppressive. CFSE-labeled CD4+CD25− lymph node Tconv cells from normal B6 mice (CD45.1+) were either cultured alone (filled curves) or with resting B6 CD4+CD25+ Tregs and resting or preactivated B6 CD4+CD25− lymph node T cells (open curves), and were stimulated to proliferate by anti-CD3 (1 μg/mL) and APCs. Preactivated B6 CD4+CD25− T cells were obtained as in panel A. The percentage of Tconv cells that failed to divide at least once in cocultures is shown. (C) Suppression by preactivated Tconv cells requires CTLA-4 externalization and expression. Preactivated or resting CD4+CD25− lymph node T cells of B6, Ctla4−/−, CTLA-4TgWT, or CTLA-4TgΔ origin were cocultured with CFSE-labeled B6 CD4+CD25− lymph node Tconv cells and stimulated to proliferate by anti-CD3 (1 μg/mL) and APCs. Lymph node T cells of Ctla4−/− and CTLA-4TgΔ origin were purified from mixed BM chimeras. In the left panel, Tconv cell proliferation was assessed by CFSE dilution. The percentage of suppression = 100*[(% of Tconv cells cultured alone that had divided at least once) − (% of Tconv cells from Treg cocultures that had divided at least once)] / (% of Tconv cells alone that had divided at least once). Means ± SE of 3 independent experiments and statistically significant differences as determined by the 2-tailed Student t test are indicated. In the right panel, CD25 surface staining on preactivated or resting CD4+CD25− T cells was quantified into mean fluorescence intensity. Data are representative of 3 independent experiments.
The suppressor function of preactivated Tconv cells depends on CTLA-4
The finding that normal Tconv cells acquired suppressor function after TCR preactivation was dramatic, so we investigated whether their suppressor function actually resulted from their externalization of CTLA-4, because high CD25 expression has been reported to suppress T cell–proliferative responses by depriving proliferating T cells of required IL-2.25 To determine whether the suppressor function of preactivated normal T cells was because of CTLA-4 or CD25, we compared preactivated CD4+CD25− Tconv cells of B6, Ctla4−/−, CTLA-4TgWT, and CTLA-4TgΔ origin, which required anti-TCR preactivation to express CTLA-4 on the cell surface (supplemental Figure 5). We found that all CD4+CD25− Tconv cell populations expressed high levels of CD25 after preactivation, with Ctla4−/− Tconv cells expressing higher CD25 levels than either B6 or CTLA-4TgWT cells (Figure 4C right panel). Therefore, if CD25-mediated IL-2 deprivation were the main basis for their suppressor function, then Ctla4−/− Tconv cells would be more suppressive than those Tconv cells. However, this was not the case, because CTLA-4–deficient Tconv cells from Ctla4−/− mice were only minimally suppressive and were significantly less suppressive than CTLA-4–sufficient Tconv cells of B6, CTLA-4TgWT, or CTLA-4TgΔ origin (Figure 4C). We conclude that the suppressor function of preactivated Tconv cells is predominantly because of external expression of CTLA-4, not CD25.
Discussion
In the present study, we assessed the basis for CTLA-4 protein function in both regulatory and conventional CD4+ T cells. CTLA-4 proteins were shown to be responsible for the 3 characteristic Treg functions of suppression, hyposignaling, and anergy. Treg suppression and anergy were both mediated by the external domain of CTLA-4, which binds to costimulatory ligands on APCs, whereas TCR hyposignaling required the internal domain of CTLA-4. The ability of CTLA-4 proteins to affect cell function differed in regulatory and conventional T cells, because transgenic CTLA-4 proteins in Tregs induced suppression, hyposignaling, and anergy, whereas the same transgenic CTLA-4 proteins in nonregulatory Tconv cells were retained in Golgi vesicles and did not affect cell function. However, TCR-signaled preactivation stimulated conventional CD4+ T cells to express and externalize CTLA-4 proteins, which imparted to these conventional T cells the ability to suppress activation of third-party naive T cells. Therefore, CTLA-4 externalization can mediate Treg suppression and impart suppressor function to any CD4+ T cell.
Tregs are functionally distinct from Tconv cells in that they are suppressive, hyporesponsive, and anergic. These 3 functions have been invariably linked in Tregs1,4,26 because, as shown in the present study, all 3 Treg functions are mediated by CTLA-4. We found that CTLA-4–deficient Tregs of Ctla4−/−Foxp3+ genetic origin lacked all 3 functions and that all 3 functions were reconstituted by expression of transgenic CTLA-4 proteins. Moreover, structure/function analyses revealed that Tregs expressing truncated CTLA-4TgΔ proteins were suppressive and anergic even though TCR signaling was not impaired, demonstrating that TCR hyposignaling was not intrinsic to either Treg suppression or Treg anergy and that neither suppression nor anergy required signaling by the internal domain of CTLA-4.
Suppression was associated with Treg externalization of CTLA-4 proteins that then interacted with costimulatory ligands on APCs. Several different mechanisms have been reported to explain how the interaction between CTLA-4 and costimulatory ligands suppresses the activation of naive T cells. Externalized CTLA-4 proteins have been shown to induce costimulatory blockade either by sequestering or removing costimulatory ligands from the APC surface.9,27,,,,–32 Externalized CTLA-4 proteins have also been reported to stimulate APCs to secrete indoleamine 2,3-dioxygenase, which limits the available tryptophan and suppresses metabolically the activation of naive T cells.33–34 Our present results are consistent with both possible mechanisms of CTLA-4–mediated suppression. However, we think that CTLA-4–mediated suppression primarily reflects costimulation blockade because Tregs suppress costimulation-dependent T-cell responses, but fail to suppress costimulation-independent T-cell responses.35 Interestingly, a CTLA-4–independent mechanism of Treg suppression has been reported that is mediated by CD25 and deprives responding T cells of required IL-2.25 Experiments reporting Treg suppression by IL-2 deprivation 25 were only performed under conditions in which responding T cells were directly costimulated by anti-CD28 mAbs instead of APCs, which circumvents CTLA-4–mediated costimulatory blockade. Consequently, the relative importance of CTLA-4 and CD25 to Treg suppression of normal APC-costimulated responses was not known previously. The results of the present study reveal that Treg suppression of APC-costimulated responses is predominantly mediated by CTLA-4 and that CD25-mediated IL-2 deprivation is only a minor mechanism of Treg suppression.
In contrast to the significant effect of CTLA-4 proteins on Treg functions, the identical CTLA-4 proteins had no effect on the function of naive Tconv cells in CTLA-4–transgenic mice. However, CTLA-4 proteins in Tregs were localized in submembrane vesicles that rapidly recycled to/from the cell surface, whereas CTLA-4 proteins in Tconv cells were retained in perinuclear Golgi vesicles. Neither CTLA-4TgWT nor truncated CTLA-4TgΔ–transgenic proteins were detected on the surface of resting Tconv cells. Golgi retention was not reversed by inducing expression of transgenic Foxp3 proteins, but was reversed by TCR signaling, suggesting that CTLA-4 externalization in Tregs might be because of TCR signaling stimulated by high-affinity interactions with in vivo self-ligands. Indeed, externalization of CTLA-4 proteins by TCR preactivation imparted to transgenic Tconv cells the ability to suppress costimulation-dependent activation of naive T cells. Most interestingly, TCR preactivation of normal (nontransgenic) CD4+ T cells also induced them to express and externalize endogenous CTLA-4 proteins and also imparted to preactivated conventional T cells the ability to suppress naive T cells. We think that expression and externalization of CTLA-4 proteins in preactivated Tconv cells dampens the pathogenicity of in vivo immune responses both in cis (by limiting the further activation of effector T cells) and in trans (by limiting the recruitment of additional antigen-specific T cells to the pool of antigen-specific effector T cells), and is especially important when Treg cell function is limiting. Indeed, this explains why deletion of Ctla4 expression specifically in Tregs8–9 results in much milder in vivo autoimmune disease and longer survival than germline deletion of Ctla4 expression in both Tregs and Tconv cells.36–37 CTLA-4 externalization by preactivated Tconv cells also explains why effector T cells from Foxp3-deficient scurfy mice somewhat suppresses CTLA-4–deficient effector T cells in vivo in mixed BM chimeras.38
Based on our present findings that Treg suppression and anergy are both mediated by the external domain of CTLA-4, these 2 functions are intrinsically linked in Tregs and reflect the effect of CTLA-4–induced costimulatory blockade on naive responding T cells and Tregs. When CTLA-4 proteins on Tregs bind to B7 ligands on APCs and block the costimulation-dependent activation of naive T cells, it is referred to as “suppression,” but when CTLA-4 proteins on Tregs bind to B7 ligands on APCs and block the costimulation-dependent activation of the Tregs themselves, it is referred to as “anergy,” Therefore, we suggest that Treg anergy is actually CTLA-4 induced “auto-suppression” and is primarily the result of CTLA-4–mediated costimulatory blockade.
Finally, our observation that the internal domain of CTLA-4 impaired proximal TCR signaling and was necessary for Treg hyposignaling is fully consistent with observations in preactivated Tconv cells that CTLA-4 proteins recruit intracellular phosphatases and impair proximal TCR signaling.13,–15,39 Although we found that impaired TCR signaling induced by the internal domain of CTLA-4 was not necessary for either Treg suppression or anergy, it reduced TCR signaled proliferation to exogenous IL-2. Indeed, the internal domain of CTLA-4 may be important for regulating Treg homeostasis, because the number of peripheral Tregs is substantially increased in mice expressing truncated CTLA-4TgΔ proteins.
In conclusion, the present study demonstrates that the internal signaling domain of CTLA-4 is not required for either Treg suppression or anergy, both of which are mediated by the external domain of CTLA-4. This study also demonstrates that CTLA-4–induced suppression and anergy are intrinsically linked functions in Tregs that result from costimulation blockade. Finally, this study demonstrates that CTLA-4 protein function depends on its localization in or near the cell membrane and that CTLA-4 externalization is sufficient to impart suppressor function to both CD4+ Tregs and TCR-activated Tconv cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Naomi Taylor for helpful discussions, Dr Arlene H. Sharpe for originally providing Ctla4−/− mice, and Dr Remy Bosselut for providing the adenosine deaminase–based transgenic vector.
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research, Bethesda, MD.
National Institutes of Health
Authorship
Contribution: X.T. designed and performed the experiments, analyzed the data, and wrote the manuscript; F.V.L., L.P., T.G., A.A., L.G., M.K., and L.F. performed the experiments; S.O.S. analyzed the data; T.L. and C.B.T. provided the materials; and A.S. analyzed the data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alfred Singer, MD, Experimental Immunology Branch, National Cancer Institute, Bldg 10, Rm 4B36, 10 Center Dr, Bethesda, MD 20892; e-mail: singera@mail.nih.gov.


![Figure 4. CTLA-4 externalization confers suppressor function to preactivated Tconv cells. (A) CTLA-4 expression in preactivated Tconv cells from B6 mice. CTLA-4 expression in resting B6 CD4+CD25+, resting B6 CD4+CD25−, and preactivated B6 CD4+CD25− cells are displayed (shown in green). Preactivated B6 CD4+CD25− T cells were purified B6 CD4+CD25− T cells that had been stimulated overnight by plate-bound anti–TCR/CD28+IL-2 (in the absence of APCs). (B) Preactivation makes Tconv cells from B6 mice suppressive. CFSE-labeled CD4+CD25− lymph node Tconv cells from normal B6 mice (CD45.1+) were either cultured alone (filled curves) or with resting B6 CD4+CD25+ Tregs and resting or preactivated B6 CD4+CD25− lymph node T cells (open curves), and were stimulated to proliferate by anti-CD3 (1 μg/mL) and APCs. Preactivated B6 CD4+CD25− T cells were obtained as in panel A. The percentage of Tconv cells that failed to divide at least once in cocultures is shown. (C) Suppression by preactivated Tconv cells requires CTLA-4 externalization and expression. Preactivated or resting CD4+CD25− lymph node T cells of B6, Ctla4−/−, CTLA-4TgWT, or CTLA-4TgΔ origin were cocultured with CFSE-labeled B6 CD4+CD25− lymph node Tconv cells and stimulated to proliferate by anti-CD3 (1 μg/mL) and APCs. Lymph node T cells of Ctla4−/− and CTLA-4TgΔ origin were purified from mixed BM chimeras. In the left panel, Tconv cell proliferation was assessed by CFSE dilution. The percentage of suppression = 100*[(% of Tconv cells cultured alone that had divided at least once) − (% of Tconv cells from Treg cocultures that had divided at least once)] / (% of Tconv cells alone that had divided at least once). Means ± SE of 3 independent experiments and statistically significant differences as determined by the 2-tailed Student t test are indicated. In the right panel, CD25 surface staining on preactivated or resting CD4+CD25− T cells was quantified into mean fluorescence intensity. Data are representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/22/10.1182_blood-2011-11-388918/5/m_zh89991289850004.jpeg?Expires=1770397512&Signature=c~edpxb8DeJ2aGREy7nWnCaL6b2D5PvTcl1mBVNeg4BIhM48Zpxq4jSRbjK7PQvCJu--VIUaulYZSk3iid4HAYlZ8d2ErYOYLs3KTvTVl0XoMOYFRNW8Fz1-dOarWZQGmfk-cmgZGDcDx3HMheLRKKr0kIlrmJcSgiyT49l8OgDWOWFsMZZB54suJYucnnvsTZbJpYNPrFfYbFLa9EisdqV2MNFZXKmOKSlsnmih978R-lPSwA3xaHzubGXggdAHUoce--omR0WwDmFC8LawQvd-HyVUF8AWE0G4ugX44O~swGPQ~Vwufw9whcQ4EvT3zctdWjGZqUEmtcBIYKgnPg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal