Abstract
Recently, the landscape of single base mutations in diffuse large B-cell lymphoma (DLBCL) was described. Here we report the discovery of a gene fusion between TBL1XR1 and TP63, the only recurrent somatic novel gene fusion identified in our analysis of transcriptome data from 96 DLBCL cases. Based on this cohort and a further 157 DLBCL cases analyzed by FISH, the incidence in de novo germinal center B cell–like (GCB) DLBCL is 5% (6 of 115). The fusion appears exclusive to GCB and was not seen in 138 non-GCB cases examined (P = .008, Fisher exact test) but was present at low incidence in follicular lymphoma (1 of 81). In all 7 cases identified, the 3′ end of the fusion consists of exons 4 and onwards of TP63. The recurrence, subtype enrichment, and the remarkably conserved nature of the TP63 portion of the fusion suggest an important functional role in the lymphomas that harbor this event.
Introduction
Chromosomal rearrangements are a hallmark of hematologic malignancy. The 3 most recurrent translocations in diffuse large B-cell lymphoma (DLBCL) were discovered by examination of karyotypes, with the partner genes identified in the 1980s and 1990s.1 Translocations involving BCL6, BCL2, and MYC are reported in 30%, 20% to 30%, and up to 10% of de novo DLBCLs, respectively.2
Recently, we and others have described the mutational landscape of DLBCL, focusing on single nucleotide variations and small insertion/deletions.3-5 Analysis of transcriptomes also offers the opportunity to identify novel fusion gene transcripts resulting from cryptic chromosomal rearrangements hitherto unsuspected from karyotype analysis, which is limited by resolution and the complexity of the chromosomal events.6
TP63 is a paralog of the tumor-suppressive transcription factor TP53.7 In contrast to TP53, TP63 is rarely mutated in malignancy.8-10 However, overexpression of ΔN-TP63, a set of TP63 isoforms lacking the transactivation (TA) domain, has been associated with malignancies of epithelial origin.11,12 TBL1XR1 encodes a protein that is part of the NCoR/SMRT transcription repressor complex.13 Recently, deletion of the TBL1XR1 gene locus has been described in DLBCL4 and primary CNS lymphoma.14 Here, we describe a novel recurrent gene fusion involving TP63 and TBL1XR1 discovered during analysis of DLBCL transcriptomes.
Methods
To identify candidate gene fusion transcripts, Trans-ABySS15 and deFuse16 were applied to the previously described3 transcriptome data generated from 96 DLBCL and 13 follicular lymphoma (FL) cases. Validation of candidate fusion transcripts was achieved using Sanger sequencing of amplicons produced by RT-PCR with primers specific for the predicted gene fusion (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Cell of origin was assigned using gene expression profiling data when available. The tally algorithm17 was applied to IHC data to assign cell of origin for the remaining biopsies (supplemental Table 2).
FISH was performed on tissue microarrays as previously described.18 Separate breakapart assays were performed for TBL1XR1 and TP63 (for probe design, see supplemental Table 3). Images, captured using the Ariol imaging system (Leica Microsystems), were scored independently by 2 persons. Quantitative RT-PCR was performed using SYBR Green and primers for wild-type TBL1XR1 and the TBL1XR1/TP63 fusion (supplemental Table 4). The study was approved by the University of British Columbia–British Columbia Cancer Agency Research Ethics Board.
Results and discussion
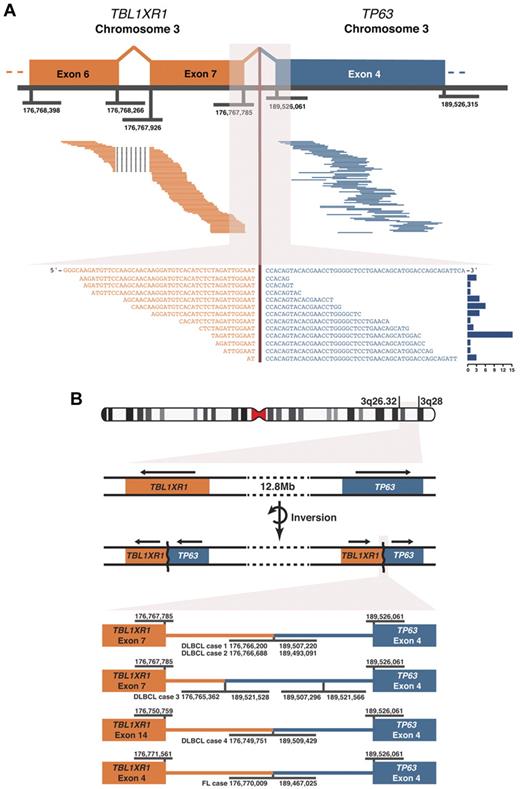
We identified evidence for fusion transcripts from massively parallel RNA sequencing (RNA-seq) of pretreatment de novo DLBCL biopsies. A novel gene fusion involving TBL1XR1 and TP63 was seen in the tumors from 4 patients (Figure 1). The existence of the TBL1XR1/TP63 fusion was validated in all 4 cases by Sanger sequencing of amplicons generated using RT-PCR. The breakpoints in the genomic DNA were identified in all cases (Figure 1B), and the genetic rearrangement was shown to be somatic by PCR and by whole genome shotgun sequencing of the constitutional DNA of one case. The TBL1XR1/TP63 fusion was the only recurrent novel somatic gene fusion observed.
TBL1XR1/TP63 gene fusion observed using paired-end massively parallel RNA sequencing and the genomic fusion breakpoints. (A) Top panel: 77 paired read sequences are shown aligning on either side of the fusion point pairing TBL1XR1 and TP63 in one case of DLBCL. Genomic coordinates of exon boundaries are shown. Bottom panel: 17 split-reads are shown that cross the fusion junction, aligned to the merged sequence of the 2 genes. The histogram on the right of this panel shows the frequency of each split-read, a total of 55 split-reads. (B) An ideogram of chromosome 3 is shown, indicating the locations of TBL1XR1 and TP63 at 3q26.32 and 3q28, respectively. The gene fusions result from chromosomal rearrangement(s) that include an inversion event. Arrows above the genes indicate the direction of transcription. At the bottom, the genomic fusion breakpoints are shown for the 5 cases detected by RNA sequencing. Genomic coordinates are given for the fusion breakpoints and the exon boundaries. In DLBCL case 3, a further inversion event is shown within intron 3 of TP63. All genomic coordinates correspond to the GRCh37 (hg19) human genome assembly.
TBL1XR1/TP63 gene fusion observed using paired-end massively parallel RNA sequencing and the genomic fusion breakpoints. (A) Top panel: 77 paired read sequences are shown aligning on either side of the fusion point pairing TBL1XR1 and TP63 in one case of DLBCL. Genomic coordinates of exon boundaries are shown. Bottom panel: 17 split-reads are shown that cross the fusion junction, aligned to the merged sequence of the 2 genes. The histogram on the right of this panel shows the frequency of each split-read, a total of 55 split-reads. (B) An ideogram of chromosome 3 is shown, indicating the locations of TBL1XR1 and TP63 at 3q26.32 and 3q28, respectively. The gene fusions result from chromosomal rearrangement(s) that include an inversion event. Arrows above the genes indicate the direction of transcription. At the bottom, the genomic fusion breakpoints are shown for the 5 cases detected by RNA sequencing. Genomic coordinates are given for the fusion breakpoints and the exon boundaries. In DLBCL case 3, a further inversion event is shown within intron 3 of TP63. All genomic coordinates correspond to the GRCh37 (hg19) human genome assembly.
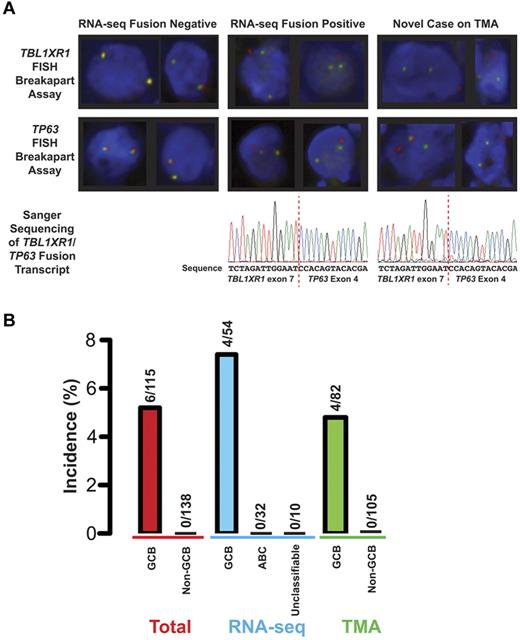
TBL1XR1 and TP63 are located 12 Mb apart on the long arm of chromosome 3, flanking the BCL6 locus. To determine the incidence of this genetic rearrangement, FISH was performed on tissue microarrays (TMAs) comprising cores of pretreatment de novo DLBCL biopsies of 187 patients. The 30-patient overlap between the RNA-seq cohort and the TMA showed that RNA-seq and FISH had 100% concordance in detecting the TBL1XR1/TP63 fusion in these samples (Figure 2A). The TMA analysis also revealed 2 new fusion-containing cases that displayed breakapart of both TBL1XR1 and TP63 loci. Sanger sequencing of amplicons generated using RT-PCR from RNA extracted from the formalin-fixed, paraffin-embedded biopsy (Figure 2A) confirmed the presence of the gene fusion in both cases. In aggregate, the incidence of the TBL1XR1/TP63 fusion was 6 of 115 (5%) cases of germinal center B cell–like (GCB) DLBCL. The fusion appeared to be exclusive to GCB DLBCL with no TBL1XR1/TP63 fusions seen in 138 cases of non-GCB DLBCL (P = .008, Fisher exact; Figure 2B).
Incidence of the TBL1XR1/TP63 fusion in DLBCL. (A) FISH and Sanger sequencing of the fusion in 3 cases of DLBCL. Left hand column: results from a case known to be negative for the fusion on analysis of RNA-seq data. Middle column: results from a case where the fusion was detected in RNA-seq data. Right hand column: results of a patient who was not part of the RNA-seq cohort. Top panels: examples of representative cells from breakapart FISH assays on TMA. These images were produced at room temperature using an Olympus BX61 microscope with a UPlanFL N 40×/0.75 objective. The fluorochromes are Spectrum green and Spectrum orange. The images were acquired using a CV-M4+CL camera (JAI) and ARIOL software (Version 3.4; Genetix). Bottom panel: electropherograms from Sanger sequencing of an amplicon that spans the junction between TBL1XR1 and TP63 in the gene fusion transcript. (B) Incidence of the TBL1XR1/TP63 fusion in the total cohort and the cohorts where the 2 detection techniques for the fusion were applied. The nonadditive nature of the cohorts toward the total is the result of an overlap of 30 DLBCLs between the 2 cohorts, including 2 DLBCLs that harbor the fusion. Cell of origin designations are as follows: total GCB, 56 by gene expression profiling and 59 by the tally algorithm; total non-GCB, 38 ABC and 14 unclassifiable by gene expression profiling and 86 non-GCB by the tally algorithm; RNA-seq, GCB, ABC, and unclassifiable are all by gene expression profiling; TMA GCB, 23 by gene expression profiling and 59 by the tally algorithm; and TMA non-GCB, 12 ABC and 7 unclassifiable by gene expression and 86 non-GCB by the tally algorithm.
Incidence of the TBL1XR1/TP63 fusion in DLBCL. (A) FISH and Sanger sequencing of the fusion in 3 cases of DLBCL. Left hand column: results from a case known to be negative for the fusion on analysis of RNA-seq data. Middle column: results from a case where the fusion was detected in RNA-seq data. Right hand column: results of a patient who was not part of the RNA-seq cohort. Top panels: examples of representative cells from breakapart FISH assays on TMA. These images were produced at room temperature using an Olympus BX61 microscope with a UPlanFL N 40×/0.75 objective. The fluorochromes are Spectrum green and Spectrum orange. The images were acquired using a CV-M4+CL camera (JAI) and ARIOL software (Version 3.4; Genetix). Bottom panel: electropherograms from Sanger sequencing of an amplicon that spans the junction between TBL1XR1 and TP63 in the gene fusion transcript. (B) Incidence of the TBL1XR1/TP63 fusion in the total cohort and the cohorts where the 2 detection techniques for the fusion were applied. The nonadditive nature of the cohorts toward the total is the result of an overlap of 30 DLBCLs between the 2 cohorts, including 2 DLBCLs that harbor the fusion. Cell of origin designations are as follows: total GCB, 56 by gene expression profiling and 59 by the tally algorithm; total non-GCB, 38 ABC and 14 unclassifiable by gene expression profiling and 86 non-GCB by the tally algorithm; RNA-seq, GCB, ABC, and unclassifiable are all by gene expression profiling; TMA GCB, 23 by gene expression profiling and 59 by the tally algorithm; and TMA non-GCB, 12 ABC and 7 unclassifiable by gene expression and 86 non-GCB by the tally algorithm.
The shared mutational landscape between GCB DLBCL and FL led us to determine the incidence of the fusion in FL. The TBL1XR1/TP63 fusion was predicted and confirmed using RNA-seq data in one of 13 FLs. FISH was performed on a TMA comprising cores of FL biopsies of 68 patients. The results, with no cases identified where both TBL1XR1 and TP63 loci displayed breakapart, indicate that the fusion is a rare event in FL.
With TBL1XR1 encoded from the negative DNA strand and TP63 from the positive DNA strand, the chromosomal rearrangement event(s) include an inversion that produces gene fusions encoded on the same strand (Figure 1B). In all cases, the identified junctions between the TBL1XR1/TP63 exons preserve the distal reading frame. Suggestive of functional significance, the TP63 portion of the gene fusion is conserved across all 7 cases, encoding exons 4 onwards. In 5 of the DLBCL cases, the 5′ junction point is the 3′ end of exon 7 of TBL1XR1, with exon 14 in the remaining DLBCL case and exon 4 in the FL case. In contrast, the predicted corollary, TP63/TBL1XR1, is disrupted in 3 of the 4 DLBCL cases examined. The mechanisms include a subsequent chromosomal translocation, deletion of the 3′ TBL1XR1 portion, and skipping of exon 8 of TBL1XR1 producing a frameshift resulting in a truncated protein.
The observation that the expression of the TBL1XR1/TP63 fusion was 5.2- ± 0.5-fold (mean ± SEM) higher than the wild-type TBL1XR1 mRNA, using quantitative RT-PCR on 3 of the DLBCL cases, suggests that there is a contextual change in the control of the TBL1XR1 promoter, which is driving the expression of this fusion.
The recurrent nature of the TBL1XR1/TP63 gene fusion, the conserved nature of the TP63 portion of the fusion, and its exclusive detection in the GCB subtype suggest a role in the pathogenesis of lymphomas harboring this event. Concomitant alterations of other genes known to be involved in the pathogenesis of DLBCL are given in supplemental Table 5. TP63 has 2 major sets of isoforms, distinguished at the N-terminus by the presence (TA-TP63) or lack (ΔN-TP63) of the TA domain, encoded by the first 3 exons.7 The TA-TP63 isoform has overlapping function with TP53 regarding induction of apoptosis in response to genotoxic stress.19 The ΔN-TP63 isoforms have a distinct function in the development and maintenance of stratified epithelial structures by contributing to self-renewal of basal epithelial cells.20-22 Lacking the TA domain, the TBL1XR1/TP63 protein may function similarly to ΔN-TP63, antagonizing the action of TP53, TA-TP63, and TA-TP73. We suspect that this may not only provide a proliferative advantage but also resistance to the genotoxic stress induced by chemotherapy.23 This is supported by the observation that 3 of the patients with DLBCL had primary refractory disease when treated with R-CHOP (supplemental Table 6). With the observation of deletion of the TBL1XR1 locus in DLBCL4 and CNS lymphoma,14 the disruption of TBL1XR1 function represents another possible mechanism of action. On activation, the E3 ligase activity of TBL1XR1 polyubiquitinates the NCoR/SMRT complex, thus targeting it for degradation, releasing transcriptional repression.24 Similarly, TBL1XR1 is involved in the polyubiquitination and degradation of the tumor oncoprotein BCL3.25
The TBL1XR1/TP63 gene fusion is predicted to give rise to a unique chimeric protein, in contrast to the deregulated expression of wild-type BCL6, BCL2, and MYC that result from the other recurrent chromosomal rearrangements in DLBCL. Although characterization of the function of this fusion protein is awaited, it raises the possibility that this protein may be a novel target for therapeutic intervention.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the National Cancer Institute Office of Cancer Genomics (contract HHSN261200800001E), the Terry Fox Foundation (grant 019001, Biology of Cancer: Insights from Genomic Analyses of Lymphoid Neoplasms), and Genome Canada/Genome British Columbia Grant Competition III (High Resolution Analysis of Follicular Lymphoma Genomes; J.M.C., R.D.G., and M.A.M.); and National Institutes of Health (grants P50CA130805-01, SPORE in Lymphoma, Tissue Resource Core PI Fisher; and 1U01CA114778 Molecular Signatures to Improve Diagnosis and Outcome in Lymphoma PI Chan). D.W.S. and K.L.T. were supported by the Terry Fox Foundation Strategic Health Research Training Program in Cancer Research at Canadian Institutes of Health Research (grant TGT-53912). R.D.M. is a Vanier Scholar (Canadian Institute of Health Research). A.J.M. is a Career Development Program Fellow of the Leukemia & Lymphoma Society. C.S. was supported by postdoctoral fellowships of the Cancer Research Society (Steven E. Drabin Fellowship) and the Michael Smith Foundation for Health Research. The laboratory work for this study was undertaken at the Genome Sciences Center, British Columbia Cancer Research Center and the Center for Translational and Applied Genomics, a program of the Provincial Health Services Authority Laboratories.
The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
National Institutes of Health
Authorship
Contribution: D.W.S. designed and performed the research, analyzed and interpreted data, and wrote the paper; K.L.M., S.R., R.D.M., F.C.C., and R.S.L. analyzed the RNA-seq data; S.B.-N. performed experiments and analyzed data; G.W.S. and K.L.T. reviewed pathology; J.M.C. curated the lymphoma database, participated in the original design of the project, reviewed the manuscript, and provided editorial input; M.A.M. and A.J.M. participated in the design of the original project and oversaw data collection and analysis; C.S. designed experiments and wrote the paper; and R.D.G. participated in the design of the original project, designed experiments, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Randy D. Gascoyne, Department of Pathology and Experimental Therapeutics, British Columbia Cancer Agency & British Columbia Cancer Research Centre, 675 W 10th Ave, Rm 5-113, Vancouver, BC, V5Z 1L3, Canada; e-mail: rgascoyn@bccancer.bc.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal