Abstract
Light-chain (AL) amyloidosis remains incurable despite recent therapeutic advances. Given the activity of the lenalidomide-alkylating agent combination in myeloma, we designed this phase 2 trial of lenalidomide, cyclophosphamide, and dexamethasone in AL amyloidosis. Thirty-five patients, including 24 previously untreated, were enrolled. Nearly one-half of the patients had cardiac stage III disease and 28% had ≥ 3 organs involved. The overall hematologic response (≥ partial response [PR]) rate was 60%, including 40% with very-good partial response or better. Using serum-free light chain for assessing response, 77% of patients had a hematologic response. Organ responses were seen in 29% of patients and were limited to those with a hematologic response. The median hematologic progression-free survival was 28.3 months, and the median overall survival was 37.8 months. Hematologic toxicity was the predominant adverse event, followed by fatigue, edema, and gastrointestinal symptoms. A grade 3 or higher toxicity occurred in 26 patients (74%) including ≥ grade 3 hematologic toxicity in 16 patients (46%) and ≥ grade 3 nonhematologic toxicity in 25 patients (71%). Seven patients (20%) died on study, primarily because of advanced disease. Lenalidomide, cyclophosphamide, and dexamethasone (CRd) is an effective combination for treatment of AL amyloidosis and leads to durable hematologic responses as well as organ responses with manageable toxicity. The trial was registered at www.clinicaltrials.gov (NCT00564889).
Introduction
Light-chain (AL) amyloidosis, the most common form of systemic amyloidoses, is characterized by multiorgan deposition of Ig light chain derived amyloid fibrils, in the context of a clonal plasma cell proliferative disorder.1-3 Outcome of patients with AL amyloidosis remains poor, especially when diagnosed with advanced organ involvement.4 Treatment approaches in AL amyloidosis target the clonal plasma cells with the aim of reducing the clonal Ig-free light-chain burden, and historically have consisted of alkylating agents combined with corticosteroids5-7 and, more recently, high-dose melphalan and stem cell transplantation.8-10 In general, treatment strategies that have been successful in the context of myeloma have proven to be beneficial in AL amyloidosis as well. To this end, both the immunomodulatory drugs (IMiDs; thalidomide and lenalidomide) and the proteasome inhibitor bortezomib have been studied as treatments for AL amyloidosis with variable degrees of efficacy and manageable toxicity.11-20 Lenalidomide was examined in two phase 2 studies, demonstrating hematologic response rates similar to that seen in myeloma and also clinically beneficial organ improvement.13,14 However, standard doses of lenalidomide have significant toxicity in amyloidosis, and combining lenalidomide with other active agents offers the potential to improve efficacy while using lower doses. The combination of lenalidomide with an alkylating agent appears to be particularly effective in patients with myeloma, as shown by results of trials using melphalan or cyclophosphamide in combination with lenalidomide and corticosteroids.21 Given the promising results seen with multiple myeloma (MM), we designed a phase 2 trial to evaluate the combination of lenalidomide, cyclophosphamide, and dexamethasone (CRd) in patients with newly diagnosed or previously treated AL amyloidosis. The goals of the study were to assess the hematologic response rate, organ response rate, and toxicity of lenalidomide, cyclophosphamide, and dexamethasone in patients with AL amyloidosis, and to assess the time to hematologic progression (TTP) and overall survival (OS).
Methods
Eligibility
Patients with biopsy proven AL amyloidosis, symptomatic and requiring therapy, and older than 18 years of age were enrolled in this trial provided they had measurable or evaluable hematologic disease defined as having one of the following: serum M-protein ≥ 1.0 g/dL, urinary M-protein excretion ≥ 200 mg in 24 hours, serum Ig-free light-chain (FLC) assay with involved FLC ≥ 10 mg/dL, and an abnormal FLC ratio.22 Patients were required to have adequate hematologic and organ function with absolute neutrophil count (ANC) ≥ 1000/μL, platelet count ≥ 75 000/μL, and serum creatinine < 3.0 mg/dL, all obtained within 14 days prior to enrollment. Patients had to have an Eastern Cooperative Oncology Group performance status of 0, 1, or 2 for inclusion in the trial. Patients were excluded from the study if they had non-AL amyloidosis or merely vascular amyloid in BM biopsy or in a plasmacytoma, or if carpal tunnel syndrome or skin purpura was the only evidence of disease. Patients with the following were excluded from the trial: clinically overt MM bone marrow plasma cells > 30%, bone lesions or hypercalcemia), uncontrolled infection, another active malignancy, New York Heart Association (NYHA) class III or IV, syncope ≤ 30 days before registration, known hypersensitivity to thalidomide, any previous use of lenalidomide, known seropositivity for HIV or hepatitis A, B, or C, or venous thromboembolic event ≤ 42 days before registration. Corticosteroid use for the treatment of nonmalignant disorders was permitted but concurrent use was restricted to the equivalent of prednisone 20 mg or less per day. Pregnant or nursing women, as well as women of childbearing potential who were unwilling to use a dual method of contraception, and men who were unwilling to use a condom were not eligible for the study. The trial was performed with approval of the Mayo Clinic Institutional Review Board in accordance with the principles of the Helsinki Declaration. The trial was registered at www.clinicaltrials.gov (NCT00564889).
Treatment schedule
The treatment schedule consisted of 4-week cycles of: lenalidomide given at 15 mg orally (PO) days 1-21; cyclophosphamide 300 mg/m2 PO given days 1, 8, and 15; and dexamethasone 40 mg PO given days 1, 8, 15, and 22 (weekly, continuously). For patients continuing on therapy, cyclophosphamide was given for a maximum of 12 cycles. After reports of second malignancies emerged in the context of lenalidomide and alkylator therapy, the study treatment duration was limited to 2 years, and patients who had already received > 24 cycles of therapy were taken off study. Thromboprophylaxis consisted of aspirin 81 or 325 mg given daily (physician discretion based on presence of bleeding symptoms), with low-molecular-weight heparin or coumadin recommended for patients with history of prior thrombotic events or in patients considered at higher risk for a thrombotic event based on presence of risk factors.
Dose adjustments were permitted based on toxicity. Lenalidomide was permanently discontinued for erythema multiforme/Stevens-Johnson syndrome ≥ grade 3, desquamating/blistering rash of any grade, any rash of grade 4 severity, grade 4 neuropathy or hypersensitivity, and grade 3 or higher bradycardia or cardiac arrhythmia. Subjects experiencing other grade 3 or greater adverse events felt related to lenalidomide or cyclophosphamide had the drug held until resolution of the adverse event and restarted at the next lower dose level. Hematologic toxicities required dose reductions of cyclophosphamide and lenalidomide, while other toxicities thought to be related to either one of the drugs only required reduction of the suspected drug. Lenalidomide was progressively reduced for other related grade 3 or higher adverse events to dose levels of 10 mg, and 5 mg administered on days 1 to 21 of a 28-day cycle. Cyclophosphamide was progressively dose reduced to 200 mg/m2 weekly, 100 mg/m2 weekly, or 100 mg/m2 once every 2 weeks. When grade III or IV adverse events occurred before day 15 of a cycle and resolved to grade II or lower severity before day 21 of the cycle, drugs were resumed at the next lower dose level until day 21, with the next cycle continuing at the reduced dose level. For grade III or IV adverse events occurring on or after day 15 of a given cycle, they were held for the remainder of the cycle and reduced by one dose level beginning with the next cycle. Once the dose of any of the drugs was reduced for toxicity, no dose re-escalation was permitted. Dexamethasone-related toxicity was managed by lowering the dose progressively to 30 mg, 20 mg, and 10 mg weekly. Patients unable to tolerate the lowest doses of any of the drugs needed to stop therapy with that agent permanently. Routine antibiotic, antiviral, or antifungal prophylaxis was not mandated and left to the discretion of the treating physician.
Response and toxicity assessment
A hematologic partial response (PR) was defined as a 50% reduction in the level of the serum monoclonal protein and/or a reduction in 24-hour urinary light-chain excretion by 90% or to < 200 mg.22 A very-good PR (VGPR) required, in addition to criteria for PR, serum and urine monoclonal protein detectable only on immunofixation but not on electrophoresis, or a 90% reduction in serum monoclonal protein and 24-hour urine monoclonal protein of < 100 mg/24 h. Complete response (CR) required complete disappearance of the monoclonal protein in the serum and urine by immunofixation studies and < 5% plasma cells on BM examination. In patients in whom the only measurable disease was by serum FLC levels, CR required a normal kappa/λ FLC ratio of 0.26 to 1.65 in addition to CR criteria listed earlier. VGPR in such patients was defined as a > 90% decrease in the difference between involved and uninvolved FLC levels. PR in such patients required a 50% decrease in the difference between involved and uninvolved FLC levels. All response categories (CR, VGPR, and PR) require 2 consecutive assessments to be considered confirmed responses.
Hematologic progression required any one of the following criteria: increase in serum monoclonal protein of 25% or higher above the lowest response level and an absolute increase by > 5 g/L or increase in urine monoclonal protein by 25% above the lowest remission value and an absolute increase in excretion by > 200 mg/24 h. In patients in whom the only measurable disease is serum FLC, the requirements were a 50% increase in the difference between involved and uninvolved FLC levels from the lowest remission level, and an absolute increase of at least 10 mg/dL.
Organ response was evaluated on the basis of improvement of one or more affected organs as previously described.23 Only one parameter was required to satisfy the organ response criteria, and the response needed to be maintained for a minimum of 3 months to be considered valid. Renal response required a 50% reduction in 24-hour urine protein excretion (at least 0.5 g/d) with stable creatinine. Cardiac response required one of (1) ≥ 2-mm reduction in the interventricular septal (IVS) thickness by echocardiogram, (2) improvement of ejection fraction by ≥ 20%, or (3) improvement by 2 NYHA classes without an increase in diuretic use. Hepatic response required either (1) ≥ 50% decrease in (or normalization of) an initially elevated alkaline phosphatase level or (2) reduction in the size of the liver by at least 2 cm by radiographic determination. A neurologic response was defined as either a reduction in the Neuropathy Impairment Score (NIS) by 10 points, or improvement in the summated compound muscle action potential (CMAP) amplitude by 2 mV. This value is derived from the summated value of CMAP amplitudes of the tibial, peroneal, and ulnar nerves from the nerve conduction studies. Gastrointestinal tract improvement was defined as (1) normalization of a low serum carotene level, (2) reduction of diarrhea to < 50% of previous movements/day, or (3) decrease in fecal fat excretion by 50%.
Organ progression was defined by fulfillment of at least one of the following criteria. Renal progression was defined as 50% increase in urinary protein loss (at least 1 g/24 h), or 25% worsening of creatinine or creatinine clearance from the lowest response level (minimum change of 0.5 mg/dL and 15 mL/min, respectively). Cardiac progression required an increase in cardiac wall thickness by ≥ 2 mm or an increase in NYHA class by one grade with a decreasing ejection fraction of ≥ 10%. A hepatic progression required either (1) ≥ 50% increase of alkaline phosphatase level above lowest confirmed level or (2) an increase in liver size by at least 2 cm (radiographic determination). A neurologic progression meant an increase in the NIS by 10 points, or worsening in the summated CMAP amplitude by 2 mVA (millivolt amps). Gastrointestinal progression was defined as a reduction of serum carotene level below normal limit or worsening of diarrhea with an increase > 50% of previous movements per day or fecal fat by 50%. All toxicities were graded and attributed according to the National Cancer Institute Common Toxicity Criteria for Adverse Events (CTCAE; Version 3). Toxicity was defined as an adverse event considered to be possibly, probably, or definitely related to treatment.
Statistical analysis
All patients meeting the eligibility criteria who signed a consent form and had begun treatment were considered evaluable for response. The primary endpoint for this trial was the proportion of patients who after 3 months had a confirmed hematologic response (CR, VGPR, or PR). The study used a modified Fleming design based on the binomial distribution to test that the true confirmed response rate was at most 5% versus the alternative that it was at least 20% with 90% power. The regimen would be declared ineffective if a maximum of 4 confirmed hematologic responses were observed in the first 32 evaluable patients. All analysis is based on the intent-to-treat principle. Duffy and Santner confidence intervals (CI) were constructed for the primary endpoint of confirmed hematologic response.
Secondary endpoints included response rate over all cycles, OS, progression-free survival (PFS), duration of response (DOR), and adverse event profile among this group of patients receiving primary therapy with the combination. Toxicities were graded according to CTCAE (Version 3.0) and the toxicity rates and depth were summarized across the cycles received with particular emphasis on potentially cumulative toxicities. OS was defined as the time between registration date and death because of any cause, with those alive censored at the date of last follow-up. Hematologic PFS was defined as the time from registration to hematologic progression or death; hematologic or organ PFS was defined from registration to hematologic or organ progression or death as the case may be. DOR was defined as the time from first response until the date of progression (or date of last follow-up in patients without progression) in the subset of patients who responded to treatment. The distribution of survival time and DOR was estimated using the method of Kaplan-Meier. Three subanalyses for OS and TTP were conducted based on cardiac biomarker staging,24 3-cycle landmark analysis including FLC measurements of hematologic response,25 and deaths on active treatment.
To estimate the time to new treatment, a competing risk analysis was performed where new treatment (time from study entry until new treatment) was the event of interest, and death (time from study entry until death) was the competing risk. Patients who were still alive without a new treatment were censored at the time of their last follow-up.
Results
The study enrolled 35 patients between December 2007 and November 2008, 24 patients who were newly diagnosed, and 11 with 1-2 prior therapies. The median age for the enrolled patients was 64 years (range; 44-82 years), and 19 (54%) were male. The baseline characteristics are detailed in Table 1. The median time from diagnosis to study enrollment was 1.6 months (range 0.1 month-10.7 years). Eleven (31%) patients had prior therapy including stem cell transplantation in 7 (20%) patients. The median number of organs involved was 2, with 10 (28%) having 3 or more organs involved. The kidney was the most common involved organ (27; 77%) followed by the heart involvement (22; 63%). The dominant organ involved in each patient is shown in Table 1. Nineteen (54%) patients were alive at last follow-up, with a median follow-up (alive patients) of 32.3 months (range 7.2-40.8 months). All patients have ended protocol treatment; 7 patients (20%) had died on study and the remaining patients had discontinued for adverse events (12; 34%), came off study per protocol (6, 18%), alternate therapy (3; 9%), progression (3; 9%), or other reasons (4; 12%). The median duration on study was 7.1 months with a median of 7 cycles of therapy administered.
Baseline patient characteristics
| . | Total, N = 35 . |
|---|---|
| Median age, y (range) | 64.0 (44.0-82.0) |
| Male sex (%) | 19 (54) |
| Eastern Cooperative Oncology Group Performance Score (%) | |
| 0 | 10 (29) |
| 1 | 14 (40) |
| 2 | 11 (31) |
| Organs involved (%) | |
| 0-1 | 16 (46) |
| 2 | 9 (26) |
| ≥ 3 | 10 (28) |
| Dominant site at diagnosis (%) | |
| Heart | 10 (28) |
| Peripheral nerve | 1 (3) |
| Skin | 3 (9) |
| Kidney | 17 (49) |
| Liver | 4 (11) |
| Previous treatment | 11 (31) |
| Previous regimens | |
| Thalidomide | 1 |
| Melphalan/dexamethasone | 4 |
| Dexamethasone | 3 |
| Melphalan | 6 |
| Prior autologous blood stem cell transplantation (%) | 7 (20) |
| Median laboratory characteristics (range) | |
| Creatinine, mg/dL | 1.2 (0.5-2.8) |
| Cardiac troponin T, ng/mL | 0.02 (0.1-0.22) |
| NT-proBNP, pg/mL | 1349.0 (0.0-25 926.0) |
| Disease stage, troponin/NT-ProBNP staging, (%) | |
| Stage 1 | 8 (23) |
| Stage 2 | 12 (34) |
| Stage 3 | 15 (43) |
| . | Total, N = 35 . |
|---|---|
| Median age, y (range) | 64.0 (44.0-82.0) |
| Male sex (%) | 19 (54) |
| Eastern Cooperative Oncology Group Performance Score (%) | |
| 0 | 10 (29) |
| 1 | 14 (40) |
| 2 | 11 (31) |
| Organs involved (%) | |
| 0-1 | 16 (46) |
| 2 | 9 (26) |
| ≥ 3 | 10 (28) |
| Dominant site at diagnosis (%) | |
| Heart | 10 (28) |
| Peripheral nerve | 1 (3) |
| Skin | 3 (9) |
| Kidney | 17 (49) |
| Liver | 4 (11) |
| Previous treatment | 11 (31) |
| Previous regimens | |
| Thalidomide | 1 |
| Melphalan/dexamethasone | 4 |
| Dexamethasone | 3 |
| Melphalan | 6 |
| Prior autologous blood stem cell transplantation (%) | 7 (20) |
| Median laboratory characteristics (range) | |
| Creatinine, mg/dL | 1.2 (0.5-2.8) |
| Cardiac troponin T, ng/mL | 0.02 (0.1-0.22) |
| NT-proBNP, pg/mL | 1349.0 (0.0-25 926.0) |
| Disease stage, troponin/NT-ProBNP staging, (%) | |
| Stage 1 | 8 (23) |
| Stage 2 | 12 (34) |
| Stage 3 | 15 (43) |
Response to therapy
Fourteen of the first 32 patients (44%) achieved a confirmed hematologic response (PR or better) within the first 3 months of treatment, satisfying the primary efficacy endpoint for the trial. Across the entire trial, a confirmed hematologic response (PR or better) was seen in 21 patients (60%) including 4 (11%) with a CR, 10 patients (29%) with VGPR, and 7 patients (20%) with a PR. Among the remaining patients, 8 had a minor response or stable disease. An organ response was seen in at least 1 organ system in 11 (32%) patients; including 8 (31%) of the 26 patients with renal involvement and 5 (23%) of the 22 patients with cardiac involvement. The median time to organ response was 4.1 months (range 0.9-12.2). Organ responses were only seen in patients with hematologic response.
Disease progression and survival
The median OS for the entire cohort was 37.8 months (95% CI 12.3-not reached [NR]) and the one and 2-year OS estimates were 71% (95% CI 57-88) and 59% (95% CI 45-78), respectively (Figure 1). The median OS was 37.8 months (95% CI 5.4-NR) and 31.2 months (95% CI 10.7-NR) for previously treated and newly diagnosed patients, respectively. The median hematologic PFS was 28.3 months (95% CI 12.1-NR); the median hematologic or organ PFS was 7.4 months (95% CI 5.4-16.1; Figure 1). The median hematologic or organ PFS was 8.4 months (95% CI 1.1-NR) and 7.0 months (95% CI 4.2-20.2) for previously treated and newly diagnosed patients, respectively. The median time to any organ progression was relatively short and reflected the impact of mixed response in some patients as well as the drawback of the current organ response criteria, where despite a hematologic response and one organ improving, another organ met criteria for progression. To overcome this problem, a competing risk analysis was performed where initiation of a new treatment was the event of interest and death was the competing risk. The cumulative incidence rates were 20%, 26%, and 36% at 6, 12, and 24 months poststudy entry, respectively, representing a 64% probability of not requiring another treatment at 2 years.
Kaplan-Meier curves showing the median hematologic or organ PFS and OS from enrollment. The median hematologic or organ PFS was 7.4 months (95% CI 5.4-16.1). The median OS for the entire cohort was 37.8 months (95% CI 12.3-NR).
Kaplan-Meier curves showing the median hematologic or organ PFS and OS from enrollment. The median hematologic or organ PFS was 7.4 months (95% CI 5.4-16.1). The median OS for the entire cohort was 37.8 months (95% CI 12.3-NR).
Overall, 12 patients (34%) have received any subsequent treatment after CRd. Subsequent therapies were used after patients went off study for toxicity (6 patients), disease progression (5 patients), and lack of adequate response (one patient). The salvage regimens used included melphalan and dexamethasone in 5 patients, bortezomib with dexamethasone in 2 patients, bortezomib, dexamethasone, and cyclophosphamide in 2 patients, and one patient each received melphalan, bortezomib, and dexamethasone, autologous stem cell transplantation, and lenalidomide.
Three-cycle landmark analysis
Given the risk of early death, we did a landmark analysis at 3 cycles from start of therapy. Eight (23%) patients did not complete 3 cycles of treatment; reasons for ending treatment early included adverse events (4 patients), death on-study (3 patients), and disease progression (one patient). When the baseline characteristics between patients who completed 3 cycles of treatment were compared with those who did not, those completing 3 cycles had a lower proportion of patients with NYHA class 2 disease (41% vs 87%, Fisher exact P = .04) and lower NT-ProBNP (median 1064 vs 6819 pg/mL, rank sum P = .03).
The hematologic response rate among the patients completing at least 3 cycles of treatment was 78%, including CR,4 VGPR,10 and PR.7 The 2-year OS estimates for patients completing at least 3 cycles of treatment and those who did not was 70% (95% CI 54-89) and 25% (95% CI 7-83), respectively. Of those patients who completed 3 cycles of treatment, 21 had at least a 50% decrease in difference between involved and uninvolved FLC (dFLC) versus 6 patients who did not have a 50% decrease. The median hematologic PFS for patients with a 50% decrease in dFLC was not attained; the median hematologic or organ PFS for these patients was 11.5 months (95% CI 3.7-NR). For patients without a 50% decrease in dFLC, the median hematologic PFS was 10.5 months (95% CI 0.3-NR) and the median hematologic or organ progression was 5.7 months (95% CI 0.3-NR).
Cardiac biomarker analysis
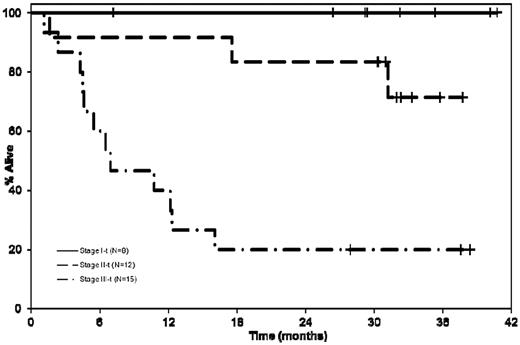
We then examined the outcome of patients with respect to the cardiac biomarker staging.24 The currently used staging system groups patients into 3 stages (stages I, II, and III), based on the presence of none, one, or both of cardiac troponin T (cTnT) > 0.035 ng/mL and NT-ProBNP > 332 pg/mL, respectively.24 The median OS was not reached for patients in stage I compared with 37.8 months (95% CI 17.5-NA) and 7 months (95% CI 4.2-12.3) for patients in stage II and III, respectively; log rank P < .001 (Figure 2). The responses and other outcomes by disease stage are as detailed in Table 2. The overall responses were lower in stage III patients, which may be a reflection of the inability to tolerate therapy in this group, given the fewer number of cycles administered and higher proportion of patients off study for adverse events as well as patients dying on study.
Kaplan-Meier curves comparing the OS of patients with respect to the cardiac biomarker staging.24 The median OS was not reached for patients in stage I compared with stage II 37.8 months (95% CI 17.5-NR), and 7 months (95% CI 4.2-12.3) for patients in stage III; log rank P < .001.
Kaplan-Meier curves comparing the OS of patients with respect to the cardiac biomarker staging.24 The median OS was not reached for patients in stage I compared with stage II 37.8 months (95% CI 17.5-NR), and 7 months (95% CI 4.2-12.3) for patients in stage III; log rank P < .001.
Relationship between cardiac biomarker staging and outcome
| . | Stage I, 8 (23%) . | Stage II, 12 (34%) . | Stage III, 15 (43%) . | Log rank P . |
|---|---|---|---|---|
| Response | ||||
| No. of responders (%) | 6 (75) | 9 (82) | 6 (40) | |
| CR | 1 | 3 | 0 | |
| VGPR | 4 | 2 | 4 | |
| PR | 1 | 4 | 2 | |
| No. of cycles | 131 | 189 | 80 | |
| Median no. of mo on therapy (range) | 13 (3-39) | 13 (1-32) | 4 (1-17) | |
| Proportion of patients with at least 1 ≥ grade III toxicity (%) | 4 (50) | 10 (83) | 12 (80) | |
| Proportion of patients with at least 1 ≥ grade III nonhematologic toxicity (%) | 4 (50) | 9 (75) | 12 (80) | |
| Patients off for adverse event | 1 | 4 | 7 | |
| Died while on active therapy | 0 | 1 | 6 | |
| Survival | ||||
| Overall survival | ||||
| Median (95% CI) | NR | NR | 7 mo (4.6-12.3) | .0001 |
| No. of events | 0 | 2 | 12 | |
| PFS (hematologic or organ progression) | ||||
| Median (95% CI) | NR | 12.0 mo (1.6-NR) | 4.5 mo (1.1-6.5) | .004 |
| No. of events | 3 | 8 | 14 | |
| PFS (hematologic progression only) | ||||
| Median (95% CI) | NR | NR | 6.8 mo (4.2-28.3) | .005 |
| No. of events | 1 | 5 | 11 |
| . | Stage I, 8 (23%) . | Stage II, 12 (34%) . | Stage III, 15 (43%) . | Log rank P . |
|---|---|---|---|---|
| Response | ||||
| No. of responders (%) | 6 (75) | 9 (82) | 6 (40) | |
| CR | 1 | 3 | 0 | |
| VGPR | 4 | 2 | 4 | |
| PR | 1 | 4 | 2 | |
| No. of cycles | 131 | 189 | 80 | |
| Median no. of mo on therapy (range) | 13 (3-39) | 13 (1-32) | 4 (1-17) | |
| Proportion of patients with at least 1 ≥ grade III toxicity (%) | 4 (50) | 10 (83) | 12 (80) | |
| Proportion of patients with at least 1 ≥ grade III nonhematologic toxicity (%) | 4 (50) | 9 (75) | 12 (80) | |
| Patients off for adverse event | 1 | 4 | 7 | |
| Died while on active therapy | 0 | 1 | 6 | |
| Survival | ||||
| Overall survival | ||||
| Median (95% CI) | NR | NR | 7 mo (4.6-12.3) | .0001 |
| No. of events | 0 | 2 | 12 | |
| PFS (hematologic or organ progression) | ||||
| Median (95% CI) | NR | 12.0 mo (1.6-NR) | 4.5 mo (1.1-6.5) | .004 |
| No. of events | 3 | 8 | 14 | |
| PFS (hematologic progression only) | ||||
| Median (95% CI) | NR | NR | 6.8 mo (4.2-28.3) | .005 |
| No. of events | 1 | 5 | 11 |
PFS indicates progression-free survival; CI, confidence interval; and NR, not reached.
Safety and tolerability
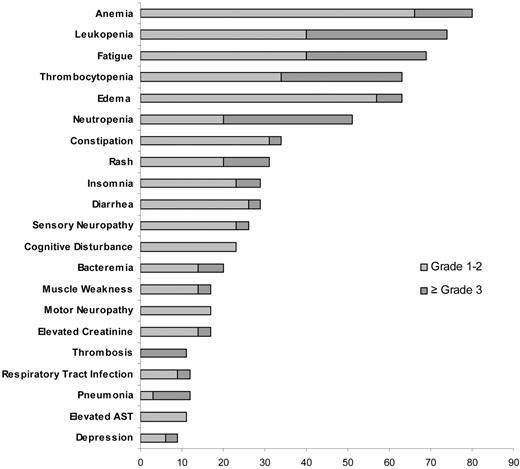
The most common toxicities at least possibly attributed to the study medication were hematologic in nature. The most common nonhematologic toxicities included pedal edema and insomnia likely related to dexamethasone and fatigue and constipation potentially related to lenalidomide. A grade 3 or higher toxicity was reported in 26 patients (74%), including grade 3 or higher hematologic toxicity in 16 patients (46%) and grade 3 or higher nonhematologic toxicity in 25 patients (71%). The common toxicities and the grades of toxicity are detailed in Figure 3. Twenty-two (63%) patients had at least one treatment delay with a total of 46 treatment delays recorded among the 400 cycles delivered across the study; the most common cause for delay was hematologic toxicity. The toxicities were manageable with dose reductions, with 97%, 75%, and 67% of the target dose delivered for cyclophosphamide, dexamethasone, and lenalidomide, respectively (Table 3).
Frequency of major hematologic and nonhematologic toxicity across the entire trial.
Frequency of major hematologic and nonhematologic toxicity across the entire trial.
Treatment administration
| Treatment administration . | . |
|---|---|
| Median no. of cycles administered (range) | 7 (1-40) |
| Total number of cycles administered | 400 |
| Lenalidomide | |
| Number of cycles administered | 400 |
| Median % of targeted dose (range) | 67 (3.2-100) |
| No. of patients with dose reduction (%) | 20 (57) |
| Total reductions | 25 |
| Reasons for reductions | |
| Hematologic adverse event | 6 |
| Dermatology/skin/rash | 9 |
| Infection | 2 |
| Other | 8 |
| Cyclophosphamide | |
| No. of cycles administered | 268 |
| Median % of targeted dose (%) | 97 (0-108.3) |
| No. of patients with dose reduction (%) | 20 (57) |
| Total reductions | 28 |
| Reasons for reductions | |
| Hematologic adverse event | 16 |
| Renal | 1 |
| Infection | 3 |
| Other | 8 |
| Dexamethasone | |
| No. of cycles administered | 384 |
| Median % of targeted dose (range) | 75 (0-100) |
| No. of patients with dose reduction (%) | 21 (60) |
| Total reductions | 44 |
| Reasons for reductions | |
| Gastrointestinal | 3 |
| Edema | 7 |
| Weakness | 3 |
| Confusion/mood alteration | 7 |
| Other | 24 |
| Treatment administration . | . |
|---|---|
| Median no. of cycles administered (range) | 7 (1-40) |
| Total number of cycles administered | 400 |
| Lenalidomide | |
| Number of cycles administered | 400 |
| Median % of targeted dose (range) | 67 (3.2-100) |
| No. of patients with dose reduction (%) | 20 (57) |
| Total reductions | 25 |
| Reasons for reductions | |
| Hematologic adverse event | 6 |
| Dermatology/skin/rash | 9 |
| Infection | 2 |
| Other | 8 |
| Cyclophosphamide | |
| No. of cycles administered | 268 |
| Median % of targeted dose (%) | 97 (0-108.3) |
| No. of patients with dose reduction (%) | 20 (57) |
| Total reductions | 28 |
| Reasons for reductions | |
| Hematologic adverse event | 16 |
| Renal | 1 |
| Infection | 3 |
| Other | 8 |
| Dexamethasone | |
| No. of cycles administered | 384 |
| Median % of targeted dose (range) | 75 (0-100) |
| No. of patients with dose reduction (%) | 21 (60) |
| Total reductions | 44 |
| Reasons for reductions | |
| Gastrointestinal | 3 |
| Edema | 7 |
| Weakness | 3 |
| Confusion/mood alteration | 7 |
| Other | 24 |
Seven patients (20%) died on study after a median of 4.3 months (range 1.1-12.3) of treatment. The causes of death were primarily cardiac related because of advanced cardiac amyloidosis in 5 patients, and possibly treatment related in the remaining 2 patients (one patient in whom death was likely related to sepsis and multiorgan failure, and another patient who developed small bowel obstruction and died postoperatively). We specifically compared the baseline characteristics of the 7 patients who died on study with the remaining 28 patients. The patients with early deaths were more likely to have a poorer performance status (2 vs 1 or 0), cardiac involvement (100%), and more severe cardiac involvement indicated by higher troponin and NT-ProBNP levels (Table 4).
Baseline characteristics and risk of death on study
| . | Died on study, N = 7 . | Did not die on study, N = 28 . | P . |
|---|---|---|---|
| Performance Score (%) | .0268 | ||
| 0 | 0 (0) | 10 (35.7) | |
| 1 | 2 (28.6) | 12 (42.9) | |
| 2 | 5 (71.4) | 6 (21.4) | |
| Organs involved (%) | .7928 | ||
| 1 | 2 (28.6) | 15 (54) | |
| 2 | 2 (28.6) | 7 (25) | |
| 3 | 2 (28.6) | 5 (18) | |
| 4 | 1 (14.3) | 1 (4) | |
| 6 | 0 (0) | 1 (4) | |
| Heart (%) | 7 (100) | 15 (53.6) | .0230 |
| Kidney (%) | 4 (57.1) | 23 (82.1) | .1589 |
| Liver (%) | 1 (14.3) | 4 (14.3) | 1.0000 |
| New York Heart Class (%) | .0424 | ||
| 1 | 1 (14.3) | 16 (57.1) | |
| 2 | 6 (85.7) | 12 (42.9) | |
| Serum troponin (ng/mL) | 0.1 (0.0-0.2) | 0.0 (0.0-0.2) | .0415 |
| BNP value (pg/mL) | 1069.0 (284.0-2682.0) | 228.0 (17.0-1772.0) | .0119 |
| NT-proBNP (pg/mL) | 11 228.0 (1285.0-15 675.0) | 988.0 (0.0-25926.0) | .0094 |
| LVEF % | 60.0 (35.0-75.0) | 63.5 (40.0-75.0) | .1859 |
| Creatinine | 1.2 (0.7-2.6) | 1.2 (0.5-2.8) | .7871 |
| . | Died on study, N = 7 . | Did not die on study, N = 28 . | P . |
|---|---|---|---|
| Performance Score (%) | .0268 | ||
| 0 | 0 (0) | 10 (35.7) | |
| 1 | 2 (28.6) | 12 (42.9) | |
| 2 | 5 (71.4) | 6 (21.4) | |
| Organs involved (%) | .7928 | ||
| 1 | 2 (28.6) | 15 (54) | |
| 2 | 2 (28.6) | 7 (25) | |
| 3 | 2 (28.6) | 5 (18) | |
| 4 | 1 (14.3) | 1 (4) | |
| 6 | 0 (0) | 1 (4) | |
| Heart (%) | 7 (100) | 15 (53.6) | .0230 |
| Kidney (%) | 4 (57.1) | 23 (82.1) | .1589 |
| Liver (%) | 1 (14.3) | 4 (14.3) | 1.0000 |
| New York Heart Class (%) | .0424 | ||
| 1 | 1 (14.3) | 16 (57.1) | |
| 2 | 6 (85.7) | 12 (42.9) | |
| Serum troponin (ng/mL) | 0.1 (0.0-0.2) | 0.0 (0.0-0.2) | .0415 |
| BNP value (pg/mL) | 1069.0 (284.0-2682.0) | 228.0 (17.0-1772.0) | .0119 |
| NT-proBNP (pg/mL) | 11 228.0 (1285.0-15 675.0) | 988.0 (0.0-25926.0) | .0094 |
| LVEF % | 60.0 (35.0-75.0) | 63.5 (40.0-75.0) | .1859 |
| Creatinine | 1.2 (0.7-2.6) | 1.2 (0.5-2.8) | .7871 |
BNP indicates brain natriuretic peptide; and LVEF, left ventricular ejection fraction.
Discussion
Treatment of AL amyloidosis remains a challenge given the heterogeneity of clinical presentation with the spectrum and degree of organ involvement significantly influencing the ability to tolerate different treatments.4,24 While the OS in this disease has improved in the recent years, > 40% of patients die within a year of diagnosis, an outcome that is dictated primarily by advanced cardiac involvement.4 This is in sharp contrast with myeloma, where the introduction of novel drugs has changed the treatment landscape leading to significantly improved survival.26,27 The poor outcome seen in patients with AL amyloidosis highlights the need to explore novel approaches to the treatment of this disease. As with myeloma, thalidomide, lenalidomide and bortezomib have been evaluated in AL amyloidosis demonstrating similar hematologic responses, but with very different patterns of toxicity underscoring the need to adapt these regimens specifically for this disease (Table 5).11-20
Results from recent clinical trials in patients with light-chain amyloidosis
| Reference . | Treatment regimen . | Newly diagnosed vs previously treated . | No. of patients . | Heme PR, % . | Heme CR, % . | Organ response, % . | Adverse events, ≥ grade 3, % . | OS from study entry . |
|---|---|---|---|---|---|---|---|---|
| Palladini et al6 | Mel 0.22 mg/d, Dex 40 mg/d, days 1-4 of 28 | Newly diagnosed, SCT ineligible | 46 | 67 | 33 | 48 | 11 | NA |
| Dispenzieri et al13 | Lenalidomide 25 mg/d, 21 of 28 days; Dex 40 mg, days 1-4, 15-18 added if no response after 3 cycles | Phase 2, mixed | 23 | 41 | NA | 23 | 86 | NA |
| Sanchorawala et al14 | Lenalidomide 25 mg/d, 21 of 28 days; Dex was added at 10-20 mg/d from days 1-4, 9-12, and 17-20 every other cycle, if no response after 3 cycles (lenalidomide dose was reduced to 15 mg after 8 patients) | Phase 2, mixed | 34 | 47 | 21 | 18 | NA | NA |
| Moreau et al17 | Mel 0.18 mg/kg/d, days 1-4 of 28; Dex 40 mg/d, days 1-4 of 28; lenalidomide 5, 10, or 15 mg, days 1-21 of 28 (MTD was 15 mg) | Phase 1/2 | 26 | 58 | 23 | 50 | NA | 54% at 2 y |
| Palladini et al16 | Lenalidomide 15 mg, days 1-21 of 28; Dex 20 mg on days 1, 8, 15, and 22 | Phase 2 (Btz, Mel refractory) | 24 | 38 | 0 | 4 | 50 | 14 mo |
| Dietrich et al28 | Mel 16 mg/m2 IV day 1; Dex 40 mg PO, days 1-4 of 28 | Retrospective, SCT ineligible | 61 | 44 | 11 | 25 | NA | 17.5 mo |
| Wechalekar12 | Cyclophosphamide 500 mg weekly, thalidomide 200 mg/d (start 100 mg/d, increased if tolerated) continuously; Dex 40 mg, days 1-4, 9-12, of 21 days (in elderly, 28 days cycle with Dex 20 mg, and cyclophosphamide 3 of 4 weeks) | Phase 2, mixed | 75 | 64 | 19 | 21 | NA | 41 mo |
| Kastritis18 | Btz 1.3 mg/m2 days 1, 4, 8, and 11; Dex dose was variable | Retrospective, mixed | 94 | 72 | 25 | 30 | NA | 76% at 1 y |
| Reece19 | Btz 1.3 mg/m2 days 1, 4, 8, and 11 (21-day cycle) | Phase 1/2, mixed | 34 | 67 | 24 | 27 | 79 | 84% at 1 y |
| Btz 1.6 mg/m2 days 1, 8, 15, 22 (35 day cycle) | 18 | 69 | 38 | 67 | 50 | 94% at 1 y | ||
| Wechalekar29 | Btz 1.3 mg/m2 days 1, 4, 8, and 11 (9 patients received variable doses of Dex) | Retrospective | 20 | 80 | 15 | 30 | 45 | NA |
| Reference . | Treatment regimen . | Newly diagnosed vs previously treated . | No. of patients . | Heme PR, % . | Heme CR, % . | Organ response, % . | Adverse events, ≥ grade 3, % . | OS from study entry . |
|---|---|---|---|---|---|---|---|---|
| Palladini et al6 | Mel 0.22 mg/d, Dex 40 mg/d, days 1-4 of 28 | Newly diagnosed, SCT ineligible | 46 | 67 | 33 | 48 | 11 | NA |
| Dispenzieri et al13 | Lenalidomide 25 mg/d, 21 of 28 days; Dex 40 mg, days 1-4, 15-18 added if no response after 3 cycles | Phase 2, mixed | 23 | 41 | NA | 23 | 86 | NA |
| Sanchorawala et al14 | Lenalidomide 25 mg/d, 21 of 28 days; Dex was added at 10-20 mg/d from days 1-4, 9-12, and 17-20 every other cycle, if no response after 3 cycles (lenalidomide dose was reduced to 15 mg after 8 patients) | Phase 2, mixed | 34 | 47 | 21 | 18 | NA | NA |
| Moreau et al17 | Mel 0.18 mg/kg/d, days 1-4 of 28; Dex 40 mg/d, days 1-4 of 28; lenalidomide 5, 10, or 15 mg, days 1-21 of 28 (MTD was 15 mg) | Phase 1/2 | 26 | 58 | 23 | 50 | NA | 54% at 2 y |
| Palladini et al16 | Lenalidomide 15 mg, days 1-21 of 28; Dex 20 mg on days 1, 8, 15, and 22 | Phase 2 (Btz, Mel refractory) | 24 | 38 | 0 | 4 | 50 | 14 mo |
| Dietrich et al28 | Mel 16 mg/m2 IV day 1; Dex 40 mg PO, days 1-4 of 28 | Retrospective, SCT ineligible | 61 | 44 | 11 | 25 | NA | 17.5 mo |
| Wechalekar12 | Cyclophosphamide 500 mg weekly, thalidomide 200 mg/d (start 100 mg/d, increased if tolerated) continuously; Dex 40 mg, days 1-4, 9-12, of 21 days (in elderly, 28 days cycle with Dex 20 mg, and cyclophosphamide 3 of 4 weeks) | Phase 2, mixed | 75 | 64 | 19 | 21 | NA | 41 mo |
| Kastritis18 | Btz 1.3 mg/m2 days 1, 4, 8, and 11; Dex dose was variable | Retrospective, mixed | 94 | 72 | 25 | 30 | NA | 76% at 1 y |
| Reece19 | Btz 1.3 mg/m2 days 1, 4, 8, and 11 (21-day cycle) | Phase 1/2, mixed | 34 | 67 | 24 | 27 | 79 | 84% at 1 y |
| Btz 1.6 mg/m2 days 1, 8, 15, 22 (35 day cycle) | 18 | 69 | 38 | 67 | 50 | 94% at 1 y | ||
| Wechalekar29 | Btz 1.3 mg/m2 days 1, 4, 8, and 11 (9 patients received variable doses of Dex) | Retrospective | 20 | 80 | 15 | 30 | 45 | NA |
PR indicates partial response; CR, complete response; OS, overall survival; PO, oral; NA, not available; Btz, bortezomib; Mel, melphalan; Dex, dexamethasone; SCT, autologous stem cell transplantation; and MTD, maximum tolerated dose.
The current trial builds upon the clinical experience so far with the IMiDs, especially lenalidomide.11,13,14,16,24 Initial trials with thalidomide were associated with significant toxicity.11 Subsequent studies explored risk-adapted strategies of combining thalidomide with cyclophosphamide and dexamethasone with reduced toxicity.12 We previously studied lenalidomide with or without dexamethasone in a group of mostly relapsed patients.13 While only 1 patient had a hematologic response to single-agent lenalidomide, the overall hematologic response was 41% with addition of dexamethasone for lack of response. In another trial of lenalidomide, with dexamethasone added for lack of response, the hematologic response rate was 45%.14 The hematologic response rate seen in the current trial of 60% compares favorably with the lenalidomide trials, reflecting added value for the cyclophosphamide. In the current trial, we chose to use 15 mg of lenalidomide based on the results of the prior trials where the 25 mg was poorly tolerated.13,14 As in the previous trials, organ responses were associated with hematologic responses. Overall, the toxicity pattern did not reflect any disadvantage to adding cyclophosphamide with similar hematologic toxicity seen here as in the previous lenalidomide trials. A recent French trial evaluated the combination of another alkylator, melphalan, with lenalidomide and dexamethasone.17 The hematologic response rate in this group of treatment naive patients was 58%, similar to CRd despite one-third of patients having received prior therapy in the current study. The OS and EFS estimates for the melphalan combination were longer than that observed with CRd, but 2 crucial differences need to be considered here. The melphalan trial had newly diagnosed patients compared with the current trial, where nearly one-third of the patients each had prior therapy. In addition, the prior trial had only included patients with performance status of 0 or 1, compared with one-third of the patients in the current trial with a performance score of 2.
How does this regimen compare with the bortezomib combinations? Kastritis et al in their case study of 94 patients reported an overall hematologic response rate of 71%, including 25% with CR and organ responses in 29%, slightly higher than that seen in the current study.18 The responses were fairly rapid with bortezomib with median time to response of < 2 months. This is different from the lenalidomide clinical trials, including the current one, where median time to hematologic response was longer. The durability of hematologic response seen with CRd is comparable with the results of the bortezomib study with median time to hematologic progression of 28.3 and 25.5 months, respectively. The 1-year OS estimate with bortezomib was 76% compared with the 71% with CRd, despite 26% and 43% of patients with Mayo Clinic Stage III patients in the 2 studies, respectively. In a phase 1 trial by Reece et al, patients with relapsed disease were treated with bortezomib at 1.6 mg/m2 weekly or 1.3 mg/m2 twice weekly.19 The overall response rates including CR rates were similar for the 2 regimens at 67% to 69%. While a direct comparison is not possible, the results seen with the CRd appear to be equivalent to those seen with bortezomib ± dexamethasone. More recently, experience with a combination of cyclophosphamide, bortezomib, and dexamethasone has been described and appears to be an active regimen in AL amyloidosis.30 Finally, the CRd regimen has the advantage of oral administration and lack of peripheral neuropathy as a common toxicity, which is of particular relevance as many of these patients may have underlying neuropathy because of amyloidosis.
What else can we learn from the current study? The results here again confirm the importance of hematologic response, especially deeper responses, on the likelihood of obtaining organ response.31 As in the previous reports, patients with a hematologic response were much more likely to have organ improvement. We have previously shown that response assessment based on free light-chain measurements is a better predictor of outcome compared with serum electrophoretic M-spike measurements.25 When the disease response was calculated using the serum-free light chain, a 50% and 90% decrease in the involved FLC was seen in 77% and 48% patients, respectively. As seen with other studies, the likelihood of organ response was higher among patients with at least a 50% decrease in the light chain with none of the patients failing to reach this threshold showing evidence of organ improvement.
Early deaths on study and early treatment discontinuation remain a considerable problem for this group of patients and have been uniformly seen in all amyloid trials.11,13,14,19,32 It appears to correlate with advanced cardiac disease and poor performance status at study entry. Whether this is further aggravated by the therapy remain unclear, but transient increase in cardiac markers have been seen across all studies using thalidomide or lenalidomide including this study and have previously been reported.33,34 The mechanisms behind this worsening remains unclear and is not seen with other treatment regimens such as melphalan and dexamethasone. The findings here, similar to those from previous trials using lenalidomide and dexamethasone, highlight the need for close monitoring and continued refinement of risk-adapted strategies for patients with advanced cardiac involvement.
So what should be the role of CRd combination in the management of AL amyloidosis? The hematologic response rate of 60% improves upon the response rates seen with lenalidomide and dexamethasone, and is comparable with that seen with older combinations like melphalan and dexamethasone as well as newer regimens like bortezomib and dexamethasone. It is lower than that observed with autologous stem cell transplantation, but only a small proportion of patients with AL amyloidosis are eligible for this approach because of advanced cardiac involvement. Importantly, nearly two-thirds of the responding patients were able to achieve a VGPR or better, thus increasing the likelihood of an organ response. The durability of response is clear from the long median time to hematologic progression and the long time to initiation of additional therapy in the majority of patients. Moreover, this regimen provides the convenience of an oral regimen without the risk of neuropathy associated with bortezomib-based treatments. The current combination was well tolerated with the reduced dose of lenalidomide at 15 mg daily. Future trials should compare the current regimen with bortezomib-based combinations with or without melphalan or cyclophosphamide.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors' sincere gratitude goes to Ann Birgin and Julie Mills for their help with patient enrollment and data management.
This work was supported in part by Hematologic Malignancies Program, National Cancer Institute CA90628 (S.K.), American Society of Clinical Oncology (ASCO) Career Development Program (S.K.K.). The clinical trial was funded in part by Celgene Corporation.
Authorship
Contribution: S.K.K. is the principal investigator for the study, and also designed the study, contributed patients, analyzed the data, and wrote the manuscript; S.R.H., F.K.B., V.R., M.Q.L., M.A.G., L.P.B., D.D., J.R.M., C.B.R., A.K.S., S.R.Z., P.R.G., J.A.L., R.F., S.J.R., S.V.R., and A.D. contributed patients and critically reviewed the manuscript; and J.A. and K.M.L. were involved with the statistical analysis.
Conflict-of-interest disclosure: R.F. has received a patent for the prognostication of MM based on genetic categorization of the disease; has received consulting fees from Medtronic, Otsuka, Celgene, Genzyme, BMS, and AMGEN; and has sponsored research from Celgene and Onyx. S.K.K. has research support for clinical trials from Celgene, Millennium, Novartis, and Genzyme and is a consultant for Merck. A.D. and M.Q.L. received clinical trial support from Celgene. The remaining authors declare no competing financial interests.
Correspondence: Shaji K. Kumar, MD, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: kumar.shaji@mayo.edu.