Abstract
MicroRNAs (miRNAs) have the potential to regulate cellular differentiation programs; however, miRNA deficiency in primary hematopoietic stem cells (HSCs) results in HSC depletion in mice, leaving the question of whether miRNAs play a role in early-lineage decisions un-answered. To address this issue, we deleted Dicer1, which encodes an essential RNase III enzyme for miRNA biogenesis, in murine CCAAT/enhancer-binding protein α (C/EBPA)–positive myeloid-committed progenitors in vivo. In contrast to the results in HSCs, we found that miRNA depletion affected neither the number of myeloid progenitors nor the percentage of C/EBPA–positive progenitor cells. Analysis of gene-expression profiles from wild-type and Dicer1-deficient granulocyte-macrophage progenitors (GMPs) revealed that 20 miRNA families were active in GMPs. Of the derepressed miRNA targets in Dicer1-null GMPs, 27% are normally exclusively expressed in HSCs or are specific for multipotent progenitors and erythropoiesis, indicating an altered gene-expression landscape. Dicer1-deficient GMPs were defective in myeloid development in vitro and exhibited an increased replating capacity, indicating the regained self-renewal potential of these cells. In mice, Dicer1 deletion blocked monocytic differentiation, depleted macrophages, and caused myeloid dysplasia with morphologic features of Pelger-Huët anomaly. These results provide evidence for a miRNA-controlled switch for a cellular program of self-renewal and expansion toward myeloid differentiation in GMPs.
Introduction
Hematopoiesis is a tightly regulated process of proliferation and differentiation of hematopoietic stem and progenitor cells (HSPCs) toward mature blood cells. Lineage commitment and differentiation of HSPCs are orchestrated by transcription factors that are expressed at specific developmental stages. For example, CCAAT/Enhancer-Binding Protein-Alpha (C/EBPA) is a master regulatory transcription factor that is not expressed in hematopoietic stem cells (HSCs), but starts to be expressed in a small fraction of multipotent progenitor (MPP) cells and increases steeply during the transition from the common myeloid progenitor (CMP) toward the GMP. C/EBPA drives granulopoiesis by controlling the expression of myeloid-specific genes.1,2
miRNAs belong to a class of small (approximately 22 nt) noncoding RNAs. The RNA-induced silencing complex–bound miRNAs bind to complementary sequences that are predominantly located in the 3′-untranslated regions of target mRNAs and regulate gene expression by transcript destabilization and inhibition of protein translation.3 Recently, the function of miRNAs in myeloid cells has been investigated using mouse models. For example, miRs-17/20/93/106 promote progenitor cell expansion by targeting Sequestosome-1–regulated pathways.4 In addition, miR-223 negatively regulates myeloid progenitor proliferation and fine-tunes granulocyte differentiation and activity.5 In addition, miR-146a inhibits the activity of both myeloid and lymphoid cell lineages and plays key roles in the regulation of inflammation.6
DICER1 is an evolutionarily conserved member of the RNase III family of endoribonucleases that is critical for processing of specific precursor hairpin sequences, the so-called pre-miRNAs, into miRNAs.7 Genetic deletion of Dicer1 in mice results in early embryonic mortality due to depletion of the Oct-4–positive pluripotent embryonic stem cell pool at embryonic day 6-7 (E6-E7).8 A floxed Dicer1 allele (Dicer1fl) has been generated that allows conditional deletion of Dicer1 in a cell type- and developmental stage–specific fashion.9 Hematopoietic lineage–specific conditional deletion of Dicer1 has revealed the involvement of miRNAs in the survival, maturation, and homeostasis of peripheral T lymphocytes and in Ab diversity and survival of B lymphocytes.10-12 In addition, conditional Dicer1 deletion in osteoprogenitors using mice that have Cre recombinase under the transcriptional control of the osterix promoter (Osx-GFP-Cre) results in myeloid dysplasia and acute myelogenous leukemia with acquired genetic abnormalities but intact Dicer1.13
Mouse primary HSCs are impaired by Dicer1 loss and are unable to reconstitute hematopoiesis.14 In addition, conditional deletion of Ars2, another gene required for miRNA biogenesis, in HSCs results in BM failure and increased apoptosis of hematopoietic cells in thymus and spleen.15 Therefore, the overall contribution of miRNAs to myeloid-lineage specification remains elusive. To address this issue, we generated a myeloid-specific, Cebpa-Cre–driven Dicer1 deleter mouse strain that also harbors a conditional CRE reporter containing a loxp-flanked stop sequence (LSL) and the enhanced yellow fluorescent protein (Eyfp) in the ROSA26 locus (R26-LSL-Eyfp).16 We show that Cebpa-Cre–driven Dicer1 deletion did not affect the numbers of myeloid-committed progenitors, but did play a critical role in the regulation of a developmental program required for normal granulocyte and monocyte/dendritic cell (DC)/macrophages in mice.
Methods
Mice and reconstitution experiments
To generate Cebpa-Cre;R26-LSL-Eyfp;Dicer1wt/fl/Dicer1fl/fl mice, we crossed mice that contain floxed Dicer1 alleles (Dicer1fl9 ; a kind gift of Dr P. A. Sharp, David H. Koch Institute for Integrative Cancer Research, Cambridge, MA) with Cebpa-Cre;R26-LSL-Eyfp reporter mice.2 Fetal livers were obtained on E13.5. Routine genotyping of Dicer1;Cebpa-Cre;R26-LSL-Eyfp mice was performed by PCR assays of DNA from tail or toe biopsies. Sequences of primers are available on request. All primers were obtained from Biolegio. For transplantation, 6- to 8-week-old recipient mice (C57Bl/6; The Jackson Laboratory) were irradiated (8.5 Gy) and tail-vein injected with fetal liver single-cell suspensions. Typically, cells from each fetal liver were transplanted into 2 recipient mice. Hematopoietic tissues were analyzed 6-10 weeks after transplantation. The percentage chimerism in hematopoietic tissue was detected by flow cytometric analysis of CD45.1 (recipient) and CD45.2 (Dicer1fl/fl and Dicer1fl/wt donor) cells in a total of 8 mice. All animal experiments were approved by the Animal Welfare/Ethics Committee of the Erasmus Medical Center.
Cell culture, colony assays, and cytospins
DCs were derived from BM cultures in the presence of GM-CSF as described previously.17 GM-CSF–induced colony formation assays with progenitors from E13.5 fetal livers were performed as described previously.4 Colonies were counted after 7 days of incubation at 37°C and 5% CO2 in a humidified atmosphere. For liquid cultures, E13.5 fetal liver cells were isolated and single-cell suspensions were grown in serum-free CellGro Stem Cell Growth Medium (CellGenix) supplemented with 1% penicillin/streptomycin and GM-CSF (10 ng/mL) at a density of 1 × 106 cells/mL for 7 days. For morphologic analysis of the cells, cytospins were stained with May-Grünwald-Giemsa and examined with a Leica DMLB microscope (100× and 40× objectives) and Leica Application Suite Version 2.7.1 R1 software.
Abs, cell staining, flow cytometry, and cell sorting
To obtain BM cell suspensions, femurs and tibias were crushed in a mortar in PBS with 5% FCS. Cells were passed through a 70-μm nylon sieve and erythrocytes were lysed. Lineage-positive (Lin+) cells were determined with Abs against the following lineage markers: CD3ϵ, CD11b, CD45R/B220, Ly-6G (Gr-1), and Ter119. To recognize HSPC populations, BM cells were stained with Abs against c-Kit, Sca-1, CD34, and FcγRII/III(CD16/32). Myeloid progenitors were defined as Lin−Sca-1−c-Kit+CD34+CD16/32low (CMPs), Lin−Sca-1lowc-Kit+CD34+CD16/32hi (GMPs), and Lin−Sca-1−c-Kit+CD34−CD16/32low (megakaryocyte/erythroid progenitor [MEPs]). For the analysis of differentiated EYFP+ myeloid cells in BM, cells were stained with anti-CD11b and anti–Ly-6G Abs. Peripheral blood obtained by submandibular bleeding was treated with erythrocyte lysis buffer and stained with Abs against CD11b, Ly-6G, and Ly-6C for the determination of granulocytes and monocytes. Spleen single-cell suspensions were stained with CD11b and Ly-6G. Macrophages were isolated from the peritoneal cavity with 1.5-mL washes using PBS/5% FCS. For identification of macrophages, the cell suspensions were stained with Abs against F4/80. To identify the in vitro expansion and differentiation toward DCs, whole BM cultures were stained with Abs against MHC class II and CD11c. To sort progenitors from BM, Lin+ cells were depleted before staining with the Mouse Hematopoietic Progenitor (Stem) Cell Enrichment Set (BD Biosciences) according to the manufacturer's protocol. A forward-side scatter gate excluded cell debris and remaining red blood cells. All sorted populations were more than 95% pure as determined by reanalysis. A full list of Abs used for flow cytometry and suppliers is given in supplemental Table 5 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Gene and miRNA expression profiling
EYFP+ GMPs were sorted into RLT buffer (QIAGEN). RNA was extracted with the RNeasy Micro Kit (QIAGEN). After one step of linear amplification with the RNA MessageAmp II aRNA Kit (Applied Biosystems/AmbionX), aRNA was labeled and hybridized on the Mouse Genome 430 Affymetrix 2.0 Array according to standard protocols. Concentrations and purity of RNA samples were determined on a NanoDrop ND-1000 spectrophotometer (Isogen Life Science). RNA integrity was confirmed on an Agilent 2100 Bioanalyzer (Agilent Technologies) with 6000 nano and pico chips. Microarray data were normalized with the Affymetrix Microarray Suite (MAS Version 5.0). All microarray data are available on the Gene Expression Omnibus under accession number GSE35844. To confirm the expression changes in some target genes, cDNA was produced from 1μg of aRNA using Superscript II (Invitrogen), and quantitative RT-PCR was performed using the QuantiTect SYBR Green PCR Kit (QIAGEN). Primers were obtained from Biolegio. The ΔCt value of Dicer1Δ/Δ and Dicer1wt/Δ versus Dicer1 wild-type (Dicer1wt) cells was calculated. The fold induction was calculated by the 2−ΔCt method.
miRNAs were isolated using the RNeasy Plus Mini Kit and RNeasy MinElute Cleanup Kit (QIAGEN) according to manufacturer's protocols. For miRNA profiling, TaqMan Array Rodent MicroRNA A Cards Version 2.0, which enables quantification of 375 mouse miRNAs and 6 controls, were used according to the manufacture's protocol for Megaplex Pools With Preamplification (Applied Biosystems) using the ABI PRISM 7900HT machine (Applied Biosystems).
Statistics
TargetScan Version 5.2 (http://www.targetscan.org) was used to identify putative miRNA targets. Profiling of mRNA expression was performed in triplicate for each experimental condition (Dicer1wt, Dicer1wt/Δ, or Dicer1Δ/Δ) and subsequently normalized with MAS5.0. Probe sets considered indistinguishable from the background signal were omitted from further analyses. Identification of the differentially expressed probe sets was performed using the false discovery rate (FDR)–corrected P values derived by Limma.18 P < .017 was considered statistically significant. We divided the necessary significance level (.05) by the number of pairwise comparisons, in our case, 3. The Kolmogorov-Smirnov test was used to infer differences between cumulative distribution functions, and P < .05 was considered significant. The Fisher exact test was used to infer enrichment of de-repression for mRNA targets from the identified miRNAs. A FDR-corrected P < .05 was considered statistically significant. All statistical analyses were performed with R Version 2.12 software (http://www.r-project.org).
Results
Cebpa-Cre–driven deletion of Dicer1 does not affect the number of HSPCs
In hematopoietic cells, Cebpa starts to be expressed at the MPP stage and defines a subpopulation that is instructed to develop toward the myeloid lineage,2 making it a suitable promoter to drive Dicer1 deletion for studying the role of miRNAs in myelopoiesis. Whereas the Cebpa-Cre;Dicer1wt/fl mice were viable and born at Mendelian ratios, the Cebpa-Cre;Dicer1fl/fl mice died rapidly after birth. This phenotype can be largely explained by the fact that the Cebpa promoter is highly active during the maturation of the respiratory epithelium in late gestation19 and deletes Dicer1. Lack of Dicer1 is detrimental to these cells, similar to Sonic Hedgehog (Shh)–Cre conditional Dicer1-knockout mice.20
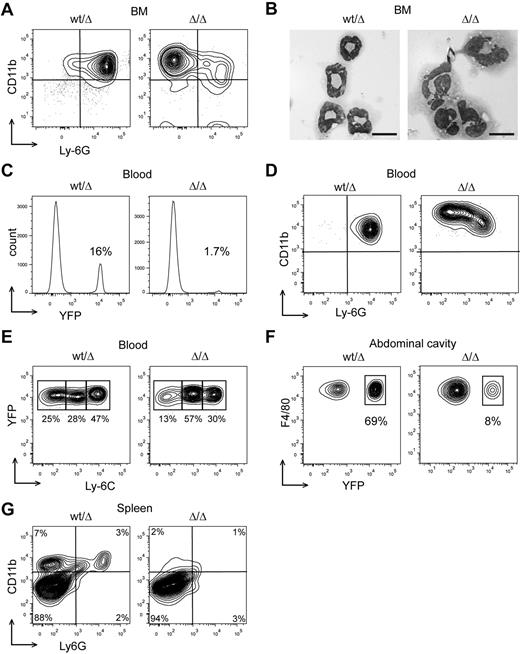
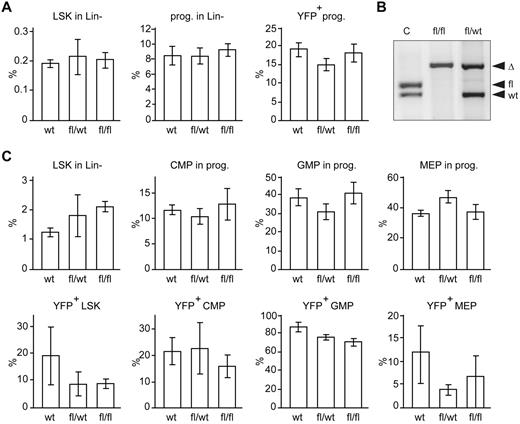
To investigate whether Dicer1 deletion affects hematopoiesis in the embryo, we isolated fetal livers at E13.5. Cells containing recombined Dicer1fl alleles (Dicer1Δ) can be identified because they also harbor a conditional CRE reporter R26-LSL-Eyfp allele.16 The E13.5 fetal livers of Dicer1 mutants and wild-type were indistinguishable by eye. In addition, flow cytometric analysis did not show any differences in the fraction of HSCs, hematopoietic progenitors, and lineage-negative (Lin−) EYFP+ progenitors in the fetal livers of Dicer1fl/fl and Dicer1wt/fl mice compared with Dicer1wt controls (Figure 1A). To investigate the effects of Cebpa-Cre–driven Dicer1 ablation in hematopoietic cells in adult mice and to bypass early death, E13.5 fetal liver cells were transplanted in lethally irradiated recipients. With this protocol, the percentage of chimerism in hematopoietic tissue was at least 92% in the reconstituted mice (data not shown). CRE-dependent deletion of Dicer1fl alleles in EYFP+ cells was confirmed by PCR (Figure 1B). Furthermore, more than 95% of total miRNAs were depleted in Dicer1Δ/Δ;EYFP+ cells (supplemental Table 1). The expression level of the remaining miRNAs was less than 10% compared with normal EYFP+ control cells (supplemental Table 1), indicating that Cebpa-Cre–mediated deletion of Dicer1 results in an efficient depletion of miRNAs in myeloid progenitor cells in vivo.
Cebpa-Cre–driven deletion of Dicer1 does not affect the fraction of myeloid-committed HSPCs in mice. (A) Percentage of LSK cells (Lin−Sca1+C-Kit+), progenitors (Lin−Sca1−C-Kit+), and EYFP+ progenitor cells of Dicer1wt (n = 3), Dicer1wt/fl (n = 4), and Dicer1fl/fl cells (n = 4) in E13.5 fetal livers. (B) EYFP+ cells from the BM of transplanted mice were sorted by flow cytometry. DNA was isolated and analyzed by PCR. DNA fragments from Cebpa-Cre;Dicer1wt/fl;R26-LSL-Eyfp and Cebpa-Cre;Dicer1fl/fl;R26-LSL-Eyfp are indicated by fl/wt and fl/fl, respectively. Tail DNA of Dicer1wt/fl was used as a positive PCR control (c) for the floxed and wild-type alleles. Recombined lox-p sites are indicated by Δ. (C) Top panel: Percentage of LSK cells (Lin−Sca1+C-Kit+), and CMPs, GMPs, and MEPs in the progenitor fraction (Lin−Sca1−C-Kit+) of Dicer1wt BM cells (n = 5), Dicer1wt/fl (n = 6), and Dicer1fl/fl cells (n = 7). Bottom panel: Percentage of EYFP+ cells in the indicated fractions.
Cebpa-Cre–driven deletion of Dicer1 does not affect the fraction of myeloid-committed HSPCs in mice. (A) Percentage of LSK cells (Lin−Sca1+C-Kit+), progenitors (Lin−Sca1−C-Kit+), and EYFP+ progenitor cells of Dicer1wt (n = 3), Dicer1wt/fl (n = 4), and Dicer1fl/fl cells (n = 4) in E13.5 fetal livers. (B) EYFP+ cells from the BM of transplanted mice were sorted by flow cytometry. DNA was isolated and analyzed by PCR. DNA fragments from Cebpa-Cre;Dicer1wt/fl;R26-LSL-Eyfp and Cebpa-Cre;Dicer1fl/fl;R26-LSL-Eyfp are indicated by fl/wt and fl/fl, respectively. Tail DNA of Dicer1wt/fl was used as a positive PCR control (c) for the floxed and wild-type alleles. Recombined lox-p sites are indicated by Δ. (C) Top panel: Percentage of LSK cells (Lin−Sca1+C-Kit+), and CMPs, GMPs, and MEPs in the progenitor fraction (Lin−Sca1−C-Kit+) of Dicer1wt BM cells (n = 5), Dicer1wt/fl (n = 6), and Dicer1fl/fl cells (n = 7). Bottom panel: Percentage of EYFP+ cells in the indicated fractions.
Previous studies showed that IFN-responsive promoter-driven Cre (Mx-Cre)–induced Dicer1 ablation results in a complete depletion of functional HSCs.14 We wondered to what extent Cebpa-driven Dicer1 deletion affects the number of myeloid-committed progenitor cells. In contrast to the results published previously for HSCs,14 in the present study, Dicer1 deletion did not affect the percentage of myeloid-committed EYFP+ cells in the LSK fraction significantly (Figure 1C and supplemental Figure 1). In addition, no significant differences in the fraction of EYFP+Dicer1Δ/Δ CMPs, GMPs, or MEPs compared with those of Dicer1wt/Δ-transplanted mice and Dicer1wt mice were observed (Figure 1C). Therefore, unlike the effect of Dicer1 deletion on HSC maintenance,14 Cebpa-Cre–driven Dicer1 deletion did not affect the numbers of myeloid progenitors in mice.
Cebpa-Cre–driven deletion of Dicer1 affects GM-CFU outgrowth, cellular replating capacity, and myeloid differentiation
We performed GM-CFU assays to determine whether deletion of Dicer1 would affect the expansion and differentiation capacity of GMPs. The number of GM-CFUs obtained with Dicer1Δ/Δ progenitor cells was approximately 50% lower than with Dicer1wt/Δ or Dicer1wt cells (Figure 2A). Colony size also decreased as a result of Dicer1 deletion (data not shown). Morphologic analyses showed a more than 5-fold increase in the number of blast-like cells, a strongly reduced capacity of the Dicer1Δ/Δ progenitors to differentiate toward macrophages, and the appearance of dysplastic neutrophils (Figure 2B). Dicer1Δ/Δ progenitor cells gained the ability to form secondary and tertiary colonies after serial replatings, which coincided with a regained self-renewal potential, a blast-like morphology, and a strongly reduced differentiation capacity of the cells (Figure 2A-B). In GM-CSF–containing liquid cultures, Lin−;Dicer1Δ/Δ progenitors were unable to mature toward macrophages, but instead showed features of dysplastic myeloid cells, including the Pelger-Huët anomaly, which is characterized by neutrophils with a hyposegmented nucleus Lin−Dicer1Δ/Δ BM cells failed to differentiate toward BM-derived DCs (Figure 2D). These results indicate that Dicer1 is essential for definitive maturation of GMPs toward both the neutrophil and monocyte/macrophage/BM–derived DC lineage in vitro.
Functional analysis of Dicer1Δ/Δ primary mouse Lin− BM cells. (A) CFU-GM assay and replating of mouse Lin− BM progenitors. Cells were plated in triplicate at densities of 1 × 104 cells per dish in 1 mL of methylcellulose medium containing GM-CSF (100 ng/mL). Cells were isolated from dishes, counted, and replated under the same conditions. Colonies consisting of more than 50 cells were counted after 7 days of growth. Significance was calculated by comparing Dicer1Δ/Δ and Dicer1wt/Δ with Dicer1wt controls using the Mann-Whitney test (asymptotic significance [2-tailed]). *P < .05. (B) Average differential cell counts (of at least 100 cells and 3 independent experiments), blast, granulocyte (-like), and macrophages. Dicer1 control plating 1 (blasts 1%, SD = 0; granulocytes 2%, SD = 1; and macrophages 97%, SD = 1). Dicer1Δ/Δ plating 1 (blasts 27%, SD = 4.7; granulocyte-like 39%, SD = 3.5; and macrophages 34%, SD = 8.1). Dicer1Δ/Δ third and fourth replatings (blasts 74%, SD = 3.6; granulocyte-like 16%, SD = 3.2; and macrophages 10%, SD = 4.6). (C) Micrographs showing cells isolated from a liquid culture of mouse Lin− BM progenitors with GM-CSF for 7 days. Arrowhead indicates the pince-nez–shaped nucleus, a hallmark for the Pelger-Huët anomaly. Black bar indicates 10 μm. (D) Number of EYFP+;CD11C+ myeloid DCs per 2 × 106 cells plated in liquid culture after 1 week of expansion (n = 3). *P < .05.
Functional analysis of Dicer1Δ/Δ primary mouse Lin− BM cells. (A) CFU-GM assay and replating of mouse Lin− BM progenitors. Cells were plated in triplicate at densities of 1 × 104 cells per dish in 1 mL of methylcellulose medium containing GM-CSF (100 ng/mL). Cells were isolated from dishes, counted, and replated under the same conditions. Colonies consisting of more than 50 cells were counted after 7 days of growth. Significance was calculated by comparing Dicer1Δ/Δ and Dicer1wt/Δ with Dicer1wt controls using the Mann-Whitney test (asymptotic significance [2-tailed]). *P < .05. (B) Average differential cell counts (of at least 100 cells and 3 independent experiments), blast, granulocyte (-like), and macrophages. Dicer1 control plating 1 (blasts 1%, SD = 0; granulocytes 2%, SD = 1; and macrophages 97%, SD = 1). Dicer1Δ/Δ plating 1 (blasts 27%, SD = 4.7; granulocyte-like 39%, SD = 3.5; and macrophages 34%, SD = 8.1). Dicer1Δ/Δ third and fourth replatings (blasts 74%, SD = 3.6; granulocyte-like 16%, SD = 3.2; and macrophages 10%, SD = 4.6). (C) Micrographs showing cells isolated from a liquid culture of mouse Lin− BM progenitors with GM-CSF for 7 days. Arrowhead indicates the pince-nez–shaped nucleus, a hallmark for the Pelger-Huët anomaly. Black bar indicates 10 μm. (D) Number of EYFP+;CD11C+ myeloid DCs per 2 × 106 cells plated in liquid culture after 1 week of expansion (n = 3). *P < .05.
Cebpa-Cre–driven deletion of Dicer1 causes myeloid dysplasia and monocyte/macrophage depletion in vivo
Next, we investigated whether the aberrant myeloid differentiation of GMPs observed in vitro also occurs in vivo. Dicer1Δ/Δ BM neutrophils showed increased levels of CD11b (a marker for myeloid cells) and a reduced Ly-6G (a marker for neutrophilic differentiation) expression compared with Dicer1wt/Δ (similar to Dicer1wt; Figure 3A). Whereas Dicer1wt/Δ neutrophils appeared normal, Dicer1Δ/Δ cells showed aberrant nucleus morphologies (Figure 3B) of which approximately 20% were hyposegmented or bilobed. Furthermore, Ly-6G+Dicer1Δ/Δ granulocytes were nearly absent in the peripheral blood and spleen (Figure 3C-D,G), suggesting that the aberrant neutrophils were incapable of emigrating from the BM.
Cebpa-Cre–driven deletion of Dicer1 causes neutrophil dysplasia and monocyte/macrophage depletion in mice. (A) FACS analysis of BM neutrophils with Abs against CD11b and Ly-6G for Dicer1wt/Δ (similar to Dicer1wt) and Dicer1Δ/Δ neutrophils. (B) Micrographs showing morphology of Dicer1wt/Δ (similar to Dicer1wt) and Dicer1Δ/Δ neutrophils. Black bar indicates 10 μm. (C) Percentage of EYFP+ cells in the blood of Dicer1wt/Δ and Dicer1Δ/Δ mice. (D) Analysis of peripheral neutrophils with Abs against CD11b and Ly-6G. (E) Analysis of peripheral EYFP+ monocytes (CD11b+ or Ly-6G−) with Abs against Ly-6C. (F) Analysis of EYFP+ macrophages from the abdominal cavity with Abs against F4/80 Ag. (G) FACS analysis of total spleen cells with Abs against CD11b and Ly6G. All analyses were performed on at least 3 mice.
Cebpa-Cre–driven deletion of Dicer1 causes neutrophil dysplasia and monocyte/macrophage depletion in mice. (A) FACS analysis of BM neutrophils with Abs against CD11b and Ly-6G for Dicer1wt/Δ (similar to Dicer1wt) and Dicer1Δ/Δ neutrophils. (B) Micrographs showing morphology of Dicer1wt/Δ (similar to Dicer1wt) and Dicer1Δ/Δ neutrophils. Black bar indicates 10 μm. (C) Percentage of EYFP+ cells in the blood of Dicer1wt/Δ and Dicer1Δ/Δ mice. (D) Analysis of peripheral neutrophils with Abs against CD11b and Ly-6G. (E) Analysis of peripheral EYFP+ monocytes (CD11b+ or Ly-6G−) with Abs against Ly-6C. (F) Analysis of EYFP+ macrophages from the abdominal cavity with Abs against F4/80 Ag. (G) FACS analysis of total spleen cells with Abs against CD11b and Ly6G. All analyses were performed on at least 3 mice.
Concerning monocytic/macrophage development, whereas Dicer1wt/Δ monocytes appeared in 3 stages of differentiation: high Ly-6C (immature), intermediate, and low Ly-6C (mature),22 the relative number of mature Dicer1Δ/Δ monocytes was strongly reduced (Figure 3E). Moreover, we observed a strongly reduced percentage of EYFP+ macrophages in the abdominal cavity of Dicer1Δ/Δ–transplanted mice compared with EYFP+;Dicer1wt/Δ controls (Figure 3F). No CD11b+ cells could be detected in the spleen of Dicer1Δ/Δ–transplanted mice (Figure 3G). Dicer1Δ/Δ recipients survived for at least 10 months devoid of any sign of myeloproliferative disease or leukemia development.
Twenty miRNA families are active in GMPs and repress expression programs characteristic of HSCs and erythropoiesis
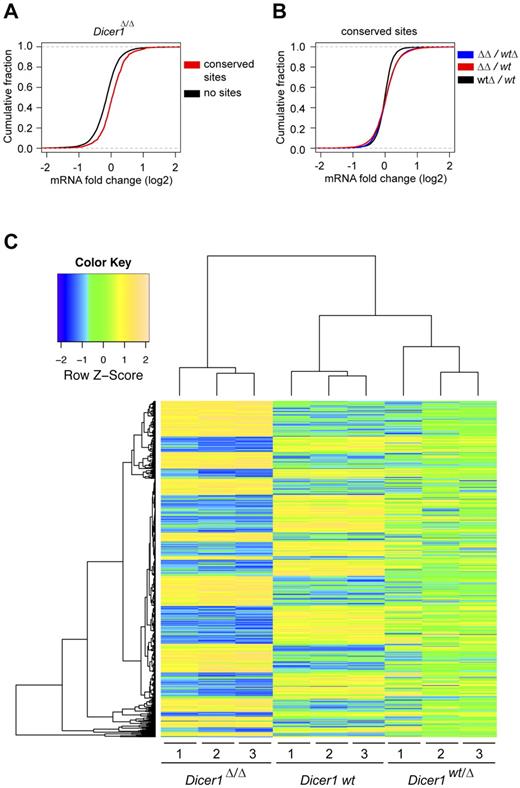
To determine how deletion of Dicer1, and the resulting loss of miRNA expression, affects the gene expression landscape of GMPs, we performed gene-expression analyses of Dicer1 mutant and control GMPs. miRNA-expression profiling of EYFP+Dicer1wt GMPs identified 104 miRNAs expressed in GMPs (supplemental Table 2). To examine the activities of these miRNAs, we compared the transcriptome of Dicer1wt, Dicer1wt/Δ, and Dicer1Δ/Δ GMPs. Transcripts with predicted binding sites for identified miRNAs tended to be up-regulated in Dicer1-null GMPs compared with transcripts without such sites (P < 2.2 × 10−16 by Kolmogorov-Smirnov test; Figure 4A), which is indicative of the activity of the miRNAs in these cells. Transcripts containing putative binding sites for the identified miRNAs had a significant propensity to be up-regulated in the Dicer1Δ/Δ cells (P < 2.2 × 10−16 by Kolmogorov-Smirnov test), but not in the Dicer1wt/Δ cells (Figure 4B). Deletion of Dicer1 in GMPs revealed 784 significant differentially expressed probe sets (FDR-corrected P < .05; Figure 4C). Unsupervised clustering of these probe sets showed that the gene-expression pattern of Dicer1wt/Δ GMPs was changed but still appeared very similar to wild-type GMPs (Figure 4C), again indicating that heterozygous deletion of Dicer1 did not strongly affect the miRNA-regulated targets in GMPs.
Cebpa-Cre–mediated Dicer1 deletion affects gene expression in GMPs. (A) Cumulative distribution plot of the log fold change of transcripts that contain miRNA-binding sites for the identified miRNAs (supplemental Table 2) and messages that do not contain sites compared with wild-type (P < 2.2 × 10−16). (B) Cumulative distribution plot of log fold change for mRNA containing miRNA-binding sites for the identified miRNAs (supplemental Table 2) in the indicated populations. The expression ratio of most messages in Dicer1Δ/Δ cells over Dicer1wt/Δ and Dicer1wt are skewed toward higher positive fold changes compared with Dicer1wt/Δ over Dicer1wt (P < 2.2 × 10−16). (C) Significant differentially expressed genes between GMP wild-type and Dicer1Δ/Δ cells (FDR-corrected P < .05) were unsupervised clustered with GPLOTS (http://cran.r-project.org/web/packages/gplots/index.html).
Cebpa-Cre–mediated Dicer1 deletion affects gene expression in GMPs. (A) Cumulative distribution plot of the log fold change of transcripts that contain miRNA-binding sites for the identified miRNAs (supplemental Table 2) and messages that do not contain sites compared with wild-type (P < 2.2 × 10−16). (B) Cumulative distribution plot of log fold change for mRNA containing miRNA-binding sites for the identified miRNAs (supplemental Table 2) in the indicated populations. The expression ratio of most messages in Dicer1Δ/Δ cells over Dicer1wt/Δ and Dicer1wt are skewed toward higher positive fold changes compared with Dicer1wt/Δ over Dicer1wt (P < 2.2 × 10−16). (C) Significant differentially expressed genes between GMP wild-type and Dicer1Δ/Δ cells (FDR-corrected P < .05) were unsupervised clustered with GPLOTS (http://cran.r-project.org/web/packages/gplots/index.html).
Cebpa-Cre–mediated deletion of Dicer1 in GMPs resulted in 300 significantly up-regulated transcripts (368 probe sets, supplemental Table 3). The up-regulation of some of these genes was confirmed by quantitative RT-PCR (supplemental Figure 2). A set of transcripts that are experimentally confirmed miRNA targets, such as Bcl2L11 (Bim),11 K-Ras and Hmga2,23 Hoxa9,24 and Cdkn1a (p21),25 also appeared to be regulated by Dicer1 in GMPs (supplemental Table 3). In agreement with data shown in Figure 4B-C, only 11 transcripts (3%) were very sensitive to Dicer1 deletion, because Dicer1 heterozygosity had a significant effect on their transcript levels (supplemental Table 3). Predicted targets of 20 miRNA families were significantly enriched in the fraction of messages that were up-regulated in Dicer1Δ/Δ cells compared with the fraction of nonregulated targets (Table 1; FDR-corrected P < .05 by Fisher exact test). Of the 300 genes that were de-repressed by Dicer1 depletion, 81 (> 25%) could be linked to signatures of HSCs, MPPs, and early erythropoiesis26 (supplemental Table 4). These findings imply that certain miRNA families control the switch of a cellular program for self-renewal and expansion toward a granulocyte/monocyte/macrophage differentiation program at the GMP stage.
Active miRNA families in GMPs
| . | Fold enrichment* . | P* . | No. of transcripts regulated . | No. of regulated transcripts linked to lineage-affiliated signatures . |
|---|---|---|---|---|
| let-7b/c/d/e/g/i | 1.84 | 2.452 × 10−03 | 55 | 9 (16.4%) |
| miR-10a | 2.42 | 1.388 × 10−02 | 16 | 3 (18.8%) |
| miR-15/16/195 | 1.65 | 1.032 × 10−02 | 57 | 8 (14%) |
| miR-17/20/93/106 | 2.82 | 2.787 × 10−10 | 83 | 15 (18.1%) |
| miR-19a/b | 1.93 | 6.528 × 10−04 | 60 | 15 (25%) |
| miR-25/92 | 2.37 | 1.657 × 10−05 | 54 | 12 (22.2%) |
| miR-26a/b | 1.80 | 8.623 × 10−03 | 45 | 10 (22.2%) |
| miR-27a/b | 1.79 | 2.452 × 10−03 | 59 | 12 (20.3%) |
| miR-30a/b/d/e | 1.82 | 1.073 × 10−03 | 69 | 14 (20.3%) |
| miR-130b/301a | 1.64 | 2.679 × 10−02 | 42 | 8 (19%) |
| miR-142-3p | 4.02 | 2.073 × 10−08 | 36 | 10 (27.8%) |
| miR-181a/c | 1.71 | 9.031 × 10−03 | 52 | 14 (26.9%) |
| miR-200c | 1.68 | 9.828 × 10−03 | 53 | 9 (17%) |
| miR-203 | 1.86 | 1.388 × 10−02 | 33 | 5 (15.2%) |
| miR-222 | 2.30 | 9.025 × 10−03 | 21 | 5 (23.8%) |
| miR-223 | 2.19 | 2.547 × 10−02 | 17 | 4 (23.5%) |
| miR-320 | 2.27 | 4.134 × 10−04 | 42 | 5 (11.9%) |
| miR-340-5p | 1.82 | 1.525 × 10−03 | 64 | 7 (10.9%) |
| miR-494 | 2.35 | 4.099 × 10−03 | 24 | 5 (20.8%) |
| miR-503 | 2.23 | 9.025 × 10−03 | 23 | 2 (8.7%) |
| . | Fold enrichment* . | P* . | No. of transcripts regulated . | No. of regulated transcripts linked to lineage-affiliated signatures . |
|---|---|---|---|---|
| let-7b/c/d/e/g/i | 1.84 | 2.452 × 10−03 | 55 | 9 (16.4%) |
| miR-10a | 2.42 | 1.388 × 10−02 | 16 | 3 (18.8%) |
| miR-15/16/195 | 1.65 | 1.032 × 10−02 | 57 | 8 (14%) |
| miR-17/20/93/106 | 2.82 | 2.787 × 10−10 | 83 | 15 (18.1%) |
| miR-19a/b | 1.93 | 6.528 × 10−04 | 60 | 15 (25%) |
| miR-25/92 | 2.37 | 1.657 × 10−05 | 54 | 12 (22.2%) |
| miR-26a/b | 1.80 | 8.623 × 10−03 | 45 | 10 (22.2%) |
| miR-27a/b | 1.79 | 2.452 × 10−03 | 59 | 12 (20.3%) |
| miR-30a/b/d/e | 1.82 | 1.073 × 10−03 | 69 | 14 (20.3%) |
| miR-130b/301a | 1.64 | 2.679 × 10−02 | 42 | 8 (19%) |
| miR-142-3p | 4.02 | 2.073 × 10−08 | 36 | 10 (27.8%) |
| miR-181a/c | 1.71 | 9.031 × 10−03 | 52 | 14 (26.9%) |
| miR-200c | 1.68 | 9.828 × 10−03 | 53 | 9 (17%) |
| miR-203 | 1.86 | 1.388 × 10−02 | 33 | 5 (15.2%) |
| miR-222 | 2.30 | 9.025 × 10−03 | 21 | 5 (23.8%) |
| miR-223 | 2.19 | 2.547 × 10−02 | 17 | 4 (23.5%) |
| miR-320 | 2.27 | 4.134 × 10−04 | 42 | 5 (11.9%) |
| miR-340-5p | 1.82 | 1.525 × 10−03 | 64 | 7 (10.9%) |
| miR-494 | 2.35 | 4.099 × 10−03 | 24 | 5 (20.8%) |
| miR-503 | 2.23 | 9.025 × 10−03 | 23 | 2 (8.7%) |
The fold enrichment and FDR-corrected P values of predicted targets in the fraction that are significantly up-regulated in DicerΔ/Δ cells compared with the nonregulated targets. Only the significant results (P < .05) of the miRNAs that are expressed in GMPs are shown. The lineage-affiliated signatures are shown in supplemental Table 4.
Discussion
The results of the present study show that Cebpa-Cre–driven Dicer1 deletion in myeloid-committed progenitors, and as a result depletion of miRNAs, disrupts the differentiation program of GMPs that is required for normal myeloid development. This has been demonstrated in 3 ways. First, normal numbers of Dicer1-null, EYFP+ myeloid-committed progenitors were detected by flow cytometry, indicating that depletion of miRNAs was not detrimental for these cells. Second, gene-expression profiling of Dicer1-null GMPs identified an altered gene expression landscape of GMPs, including enhanced expression of a set of genes that is characteristic for HSCs, MPPs, and early erythropoiesis. These data imply a disordered differentiation program in Dicer1-null GMPs. Finally, flow cytometric analysis of myeloid cells from different hematopoietic tissues in mice showed a developmental block of monocytes, strong reduction of mature macrophages in the abdominal cavity, depletion of myeloid cells in the spleen, and the presence of dysplastic neutrophils in the BM.
Recently, Raaijmakers et al reported that deletion of Dicer1 specifically in mouse osteoprogenitors disrupts normal hematopoiesis, resulting in myelodysplastic syndrome and secondary leukemias in mice.13 In these experiments, Dicer1 was not deleted in HSPCs or in the myelodysplastic cells, indicating that the observed dysplasia was initiated by osteoprogenitor dysfunction.13 Complementary to those findings, we demonstrate herein a myeloid progenitor cell–intrinsic role for miRNA processing in myelopoiesis in a system that leaves the BM environment intact. Our results indicate that Dicer1 may play dual roles in the control of myelopoiesis, both of which are essential for normal myelopoiesis.
Pelger-Huët is characterized by abnormal nuclear shape and chromatin organization in blood granulocytes.21 A genome-wide linkage scan identified the lamin B receptor (LBR), a member of the sterol reductase family located on the linked genomic region 1q41-43, to be mutated in patients suffering from Pelger-Huët disease.27 These mutations result in decreased expression of LBR, which is strongly correlated with hyposegmentation of the nucleus in neutrophils.27,28 In our model, Dicer1-null neutrophils were hyposegmented, but LBR expression remained unchanged in Dicer1-null GMPs. This result suggests that other, as-yet-undefined mechanisms may be involved in the observed developmental abnormalities or that downstream LBR pathways are controlled by Dicer1 and may cause features of Pelger-Huët anomaly.
In contrast to our results in GMPs, recently published data show that Dicer1 deletion in hematopoietic cells from different origins causes cell death due to derepression of mRNAs coding for proteins involved in the induction of apoptosis. For example, Dicer1 ablation in HSCs depletes functional HSCs, induces rapid apoptosis in HSPCs, and results in total disruption of hematopoiesis.14 These deleterious effects of miRNA depletion in HSCs can be largely circumvented by the reintroduction of a single miRNA, miR-125a, which targets the proapoptotic protein BAK1.14 In addition, Dicer1 ablation in early B-cell progenitors induces apoptosis at the pre-B cell state because of de-repression of the miR-17-92 proapoptotic target Bcl2L11 (also known as Bim) as the consequence of Dicer1 loss.11 Strikingly, although Bcl2L11 is approximately 1.8-fold up-regulated in Dicer1-null GMPs (supplemental Table 3), this did not induce an increase in apoptosis. In T cells, Dicer1 is also essential for cell viability,10,12,29 suggesting that Dicer1-dependent RNAs in HSCs and cells from lymphoid origin regulate cell survival, which agrees with our data in myeloid-committed progenitors. In addition to the regulation of apoptosis, Dicer1 plays a pivotal role in the regulation of activation, migration, lineage choice, and differentiation of T cells.10,12,29 To our knowledge, this is the first report describing an exclusive role for Dicer1 in the developmental switch of myeloid-committed progenitors toward mature neutrophils, macrophages, and myeloid DCs.
A limitation of the Dicer1-deletion models is the global depletion of miRNAs that presumably results in disruption of many cellular pathways simultaneously, which hampers the analysis of individual miRNA functions. Our data showed that Cebpa-Cre–driven Dicer1 ablation in GMPs depleted 20 active miRNA families simultaneously, resulting in de-repression of at least 300 potential miRNA targets in GMPs. Because some miRNAs regulate expression by translational inhibition without affecting mRNA stability to a detectable level, the determined level of miRNA activity is most likely an underestimation. In addition, transcripts can be regulated by multiple miRNAs, and the action of miRNAs is dependent on both miRNA and target gene levels, which complicates the functional analysis of single miRNAs in this model.30 Despite the limitations of our model, a set of miRNA target genes in GMPs is normally exclusively expressed in HSCs and, when derepressed due to Dicer1 deletion in GMPs, might explain, at least in part, some of the phenotypic features of the Dicer1-null GMPs. For example, HOXA9, a confirmed target of miR-126,24 is up-regulated in Dicer1-null GMPs, and forced expression in myeloid progenitors blocks differentiation and results in enhanced replating capacity.31 In addition, the Let-7 target HMGA223 is strongly up-regulated by Dicer1 depletion in GMPs, and overexpression or truncation of HMGA2 has been found in patients with myelodysplastic syndromes.32 In addition, the robust de-repression of the HSC-specific genes HMGA2 (8-fold) and HOXA9 (3-fold), instead of the moderate regulation shown for most miRNA targets, suggests a miRNA-driven, switch-like transition from stem cell fate toward differentiation, as shown for miRNAs lin-4 and let-7 targeting the genes lin-14 and lin-41, respectively, in Caenorhabditis elegans.33 Therefore, our data suggest that the inability to silence stem cell genes in myeloid-committed progenitors interferes with the switch of stemness toward a myeloid differentiation program. Although these findings suggest that some miRNAs function as myeloid progenitors, an extensive miRNA add-back screen in Dicer1-null cells is needed to identify their specific role in the control of myeloid development, and this one of the challenges in the field.
In conclusion, this study has demonstrated that Dicer1 ablation by C/ebpa-Cre does not affect the numbers of HSCs, CMPs, and GMPs, but results in defective GMPs, which are unable to mature toward monocytes, macrophages, and myeloid DCs, and leads instead to neutrophil dysplasia. We have identified a set of 20 highly active miRNA families in GMPs and provided evidence that Dicer1 controls a gene-expression program that is normally active in HSCs and MPPs and counteracts the expression of messages that are linked to early erythropoiesis. Our data uncover a Dicer1-controlled differentiation program in GMPs that is required for normal myelopoiesis.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr P. Leenen for providing Abs; Prof Dr P. A. Sharp for the Dicer1 floxed mice; N. Papazian, Dr K. van Lom, P. van Strien, A. Prins, and Dr E. Rombouts for technical assistance; Prof Dr H. Delwel, M. Huston, and Dr F. Cornelissen for critical reading of the manuscript; and E. Simons for assistance with figure preparation.
This work was supported by grants from The Netherlands Organization for Scientific Research and the Dutch Cancer Society.
Authorship
Contribution: M.F.A. and S.J.E. designed and performed the research, analyzed the data, and wrote the manuscript; N.G.J.A.v.B., H.W.J.d.L., and I.J.v.d.B. performed the cellular and in vivo research; M.A.S. performed the bioinformatic analysis; and T.C. and I.P.T. designed the research and discussed the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stefan J. Erkeland, PhD, Department of Hematology, Erasmus MC, Dr Molewaterplein 50, 3015 GE Rotterdam, The Netherlands; e-mail: s.erkeland@erasmusmc.nl.

![Figure 2. Functional analysis of Dicer1Δ/Δ primary mouse Lin− BM cells. (A) CFU-GM assay and replating of mouse Lin− BM progenitors. Cells were plated in triplicate at densities of 1 × 104 cells per dish in 1 mL of methylcellulose medium containing GM-CSF (100 ng/mL). Cells were isolated from dishes, counted, and replated under the same conditions. Colonies consisting of more than 50 cells were counted after 7 days of growth. Significance was calculated by comparing Dicer1Δ/Δ and Dicer1wt/Δ with Dicer1wt controls using the Mann-Whitney test (asymptotic significance [2-tailed]). *P < .05. (B) Average differential cell counts (of at least 100 cells and 3 independent experiments), blast, granulocyte (-like), and macrophages. Dicer1 control plating 1 (blasts 1%, SD = 0; granulocytes 2%, SD = 1; and macrophages 97%, SD = 1). Dicer1Δ/Δ plating 1 (blasts 27%, SD = 4.7; granulocyte-like 39%, SD = 3.5; and macrophages 34%, SD = 8.1). Dicer1 Δ/Δ third and fourth replatings (blasts 74%, SD = 3.6; granulocyte-like 16%, SD = 3.2; and macrophages 10%, SD = 4.6). (C) Micrographs showing cells isolated from a liquid culture of mouse Lin− BM progenitors with GM-CSF for 7 days. Arrowhead indicates the pince-nez–shaped nucleus, a hallmark for the Pelger-Huët anomaly. Black bar indicates 10 μm. (D) Number of EYFP+;CD11C+ myeloid DCs per 2 × 106 cells plated in liquid culture after 1 week of expansion (n = 3). *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/20/10.1182_blood-2011-10-386359/4/m_zh89991288850002.jpeg?Expires=1765933237&Signature=qeaXcFoAp8O~ts~QXWfaAMGmWxiSIZaL01f-09EewTpbOm5DnloBC5SypQPKiKqby67C4lSt52gndnabbRggjTmnYRj7nUG0uq2chTqMhAbq5aWMr4WJUttUrJbHmsp5KF5yFEk8kkTm8cnLUatj70-pGcjdrQensZx9XW6BXS-7YUYQuCZ5ctbHwLZsvkA0L5AzNzF8poKGV5~6cDWlyWeF5gq~DM3sU3bqwt~5fYOEZ7-4YNCI0EBWJzVA4FA~a6Ea0TiJSo7VwttnZiehZUBSJG7XNTa2UOEsEtZ--RBA7W7NepNH4wjlMIl0XYWVLolIhEg~vQSQDHozPnK1Ew__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)