Abstract
Chromatin remodeling is fundamental for B-cell differentiation. In the present study, we explored the role of KAP1, the cofactor of KRAB-ZFP transcriptional repressors, in this process. B-lymphoid–specific Kap1-KO mice displayed reduced numbers of mature B cells, lower steady-state levels of Abs, and accelerated rates of decay of neutralizing Abs after viral immunization. Transcriptome analyses of Kap1-deleted B splenocytes revealed an up-regulation of PTEN, the enzymatic counteractor of PIK3 signaling, and of genes encoding DNA-damage response factors, cell-cycle regulators, and chemokine receptors. ChIP/seq studies established that KAP1 bound at or close to several of these genes and controlled chromatin status at their promoters. Genome wide, KAP1 binding sites lacked active B cell–specific enhancers and were enriched in repressive histone marks, further supporting a role for this molecule in gene silencing in vivo. Likely responsible for tethering KAP1 to at least some of these targets, a discrete subset of KRAB-ZFPs is enriched in B lymphocytes. Our results therefore reveal the role of KRAB/KAP1–mediated epigenetic regulation in B-cell development and homeostasis.
Introduction
Epigenetics play a major role in ontogeny and cell specification, as illustrated by human developmental diseases caused by mutations in components of the epigenetic machinery and by the phenotypic consequences of the knockout (KO) of epigenetic regulators on mouse embryogenesis and stem cell biology.1 This level of regulation is also key to the lineage differentiation of adult tissues, in particular for the development of immune cells. As a corollary, altered epigenetic regulation has been linked to autoimmune and allergic diseases and to hematopoietic malignancies.2
B-cell progenitors (Pro-B cells) derive from multipotent lymphoid progenitors in the bone marrow (BM). Pro-B cells initiate the IgH μ locus rearrangement, completed during maturation to B-precursor (Pre-B) cells. After rearrangement of an Ig light chain locus and surface expression of a complete IgM molecule, immature B cells egress from the BM and migrate to the spleen, where they undergo further maturation steps called transitional stages 1 and 2 (T1 and T2) and eventually differentiate into mature follicular (FO) and marginal zone (MZ) B cells.3,4 FO cells are mostly composed of conventional B2 cells, which can recirculate and are responsible for T cell–dependent Ab production. In contrast, MZ B lymphocytes are sessile, nonconventional cells that, together with B1 cells, contribute to the T cell–independent Ab response. B1 cells predominantly reside in the peritoneal and pleural cavities and are thought to arise from a self-renewing fetal precursor located in the peritoneum or from B1 progenitors in adult BM.4
B-cell development and homeostasis require the integration of external and internal clues. External stimuli are sensed mainly by the BCR and other membrane receptors (ie, IL-7R, Notch2, BAFFR, and various chemokine receptors). Signals from these receptors are then conveyed through the PLCγ, Ras, STAT, and PI3K pathways and integrated within a network of transcription factors, including E2A, EBF1, and Pax5 at early stages of B-cell development and NF-κB and Aiolos in mature peripheral B cells.3,5 Recent studies have revealed that the target accessibility of these cell fate–determining transcription factors is strictly controlled by chromatin modifiers that induce the relaxation or the compaction of chromatin at these loci. In addition, chromatin remodeling is involved in regulating BCR locus rearrangement, class-switch recombination (CSR), and somatic hypermutation, all of which are key steps in B-cell development and function.6
Krüppel-associated box zinc finger proteins (KRAB-ZFPs) constitute a vast family of tetrapod-specific transcription repressors that underwent a marked expansion by gene and segment duplication during evolution.7,8 KRAB-ZFPs are characterized by the presence at their C-terminus of tandem repeats of C2H2 zinc fingers, which give them the ability to bind specific polynucleotidic sequences, and at their N-terminus of 1 or 2 KRAB domains responsible for interacting with KRAB-associated protein 1 (KAP1).9 KAP1, also known as TRIM28 or TIF1β, is a ubiquitously expressed member of the tripartite motif-containing (TRIM) family. It recruits chromatin modifiers, including SETDB1 histone methyltransferase, the CHD3/Mi2 component of the NuRD complex, and heterochromatin protein 1 (HP1), thus inducing heterochromatin formation by histone 3 trimethylation on lysine 9 (H3K9me3) and histone deacetylation.10-12 This rather advanced characterization of the biochemical mechanism of KRAB-ZFP/KAP1 action contrasts with the paucity of information on the physiologic roles of this system. Nevertheless, it has been found that KAP1 is essential for early embryonic development because during this period it participates in the pluripotent self-renewal of embryonic stem cells, the repression of endogenous and some exogenous retroviruses, and, together with KRAB-ZFP ZFP57, in the maintenance of imprinting marks. In addition, KAP1 and/or specific KRAB-ZFPs have been demonstrated to play roles in tumorigenesis, the control of behavioral stress, and Parkinson disease.13-20
In the present study, we used a conditional KO approach to investigate the impact of KRAB/KAP1-mediated regulation on B-cell development. B cell–specific Kap1-deleted mice exhibited a significant defect in B-cell differentiation and long-term Ab production. Through a combination of microarrays and ChIP sequencing, we identified several KAP1 target genes, among them Pten, the dysregulation of which likely played an important role in the observed phenotype. Genome wide, we also found that KAP1 binding sites significantly correlated with regions marked by the repressive histone modification H3K9me3 and the lack of B cell–specific regulatory elements bearing active marks such as H3K4me1 and H3K4me3 or bound by the PU.1 transcription factor.21 Finally, we identified a subset of KRAB-ZFPs enriched in B lymphocytes that are likely responsible for recruiting KAP1 to some of its genomic targets. Our data reveal the importance of KRAB/KAP1-mediated epigenetic regulation in B-cell differentiation and homeostasis.
Methods
Ethics statement
All animal experiments were approved by the local veterinary office and carried out in accordance with the European Community Council Directive (86/609/EEC) for the care and use of laboratory animals.
Mice
Generation and genotyping of mice with a floxable Kap1 allele (Kap1flox; Tif1βL3/L3), the CD19-Cre and the Cre-reporter stopfloxYFP mouse strains, have been described previously.13,22,23 CD19-Cre/Kap1flox and CD19-Cre/Kap1flox/stopfloxYFP mice were generated in a mixed C57/bl6-129sv background or C57/bl6 where specified. The offspring resulting from all of the generated strains were born at the expected rate. For a description of BM chimeras, see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Flow cytometric analysis
At the time the mice were killed, spleens were harvested, BM was obtained by flushing femur(s), and peritoneal exudate cells (PECs) by intraperitoneal wash. For the Abs used, see supplemental Methods. Cells were analyzed with a Dako CyAn ADP cytometer (Dako) or sorted with a FACSAria II (BD Biosciences). Approximately 300 000 events falling in the physical parameters gate were acquired. See Results and supplemental Methods for staining strategies.
Small-scale DNA, RNA, and protein analysis
Single cell suspensions from spleen or thymus, either total or sorted by flow cytometry where indicated, were pelleted and DNA was isolated with the DNeasy purification kit (QIAGEN) and RNA with the RNeasy Plus purification kit (QIAGEN) and reverse transcribed by Superscript II (QIAGEN), and protein obtained after lysis with RIPA buffer. For detailed protocols, see supplemental Methods. All of the primers used in this work were designed with Primer Express 3.0 software (Applied Biosystems) or the GetPrime resource (http://updepla1srv1.epfl.ch/getprime; Gubelmann et al, in press) and are listed in supplemental Methods. Specificity of all primer pairs was confirmed by dissociation curve analysis and efficiency by performing reactions with serially diluted samples.
Other assays
All microarray and ChIP/seq data are available on the Gene Expression Omnibus under accession number GSE36880. For in vitro assays, immunizations, and high-throughput analyses, please see supplemental Methods.
Statistical analysis
Nonparametric tests were used for experiments with n < 100. The Mann-Whitney test was used for comparisons between 2 groups, and Kruskal-Wallis and 2-way ANOVA for comparisons among more than 2 groups. The Spearman correlation test was used for correlation analyses and a modified Fisher exact test as a contingency test. Unless specified, 2-tailed tests were used. For statistical analysis of high-throughput data, see supplemental Methods.
Results
KAP1 controls B-lymphoid differentiation
To evaluate KRAB-ZFP/KAP1 role in B-lymphoid development and function, we crossed Kap1flox (Tif1βL3/L3)13 in a mixed C57/bl6-129sv background with the C57/bl6 heterozygous CD19-Cre mouse strain, in which the recombinase is expressed at the pro-B stage.22 CD19-Cre/Kap1flox–deficient mice were born at the expected Mendelian ratio and appeared normal and healthy. PCR-based genomic DNA analyses of CD19+ and CD19− cells confirmed that, as expected, Kap1 was deleted only in the CD19+ subset. Nevertheless, the efficiency of depletion did not reach 100% and some residual KAP1 mRNA and protein could be detected in B-enriched splenocytes (supplemental Figure 1A).
H&E staining of BM, spleen, and peripheral lymph nodes revealed normal histological features in B-lymphoid Kap1-deleted mice (data not shown). We next performed flow cytometric analyses (Figure 1A). We did not observe significant differences in the frequency of precursor (Pre; CD93+IgM−), immature (Imm; CD93+IgM+), and mature (Mat; CD93−IgM+) B cells, nor in the number of total B cells in the BM of CD19-Cre/Kap1flox–deficient compared with littermate wt/Kap1flox control mice. However, the analysis of peripheral B cells revealed a significant decrease in FO (IgMloIgDhiCD21intCD23+) and MZ (IgMhiIgDloCD21hiCD23−) B cells and an expansion of transitional stage II follicular progenitor (T2-FP; IgMhiIgDhiCD21intCD23+) B cells in the absence of KAP1. Transitional MZ progenitor (T2-MZ; IgMhiIgDhiCD21hiCD23+) and transitional stage I (T1; IgMhiIgDlo CD21lo−CD23−) cells were unaffected. Consistent with the observed reduced frequency of mature B cells, Kap1-deleted mice harbored significantly fewer B splenocytes than control littermates. Moreover, PECs from KO animals comprised a significantly reduced fraction of both B1a (CD11b+IgMhiIgDloCD5+) and B1b (CD11b+IgMhiIgDloCD5−) cells. Because a slight decrease in MZ and B1 cells has been linked to the reduced expression of the CD19 gene in which Cre is knocked in,24 we also performed flow cytometric analysis on C57/bl6 CD19-Cre/Kap1flox–deficient mice. This analysis confirmed that these animals exhibited a reduction in mature conventional and nonconventional B cells compared with their C57/bl6 CD19-Cre/KAP1wt counterparts, ascertaining that the observed phenotype was truly due to Kap1 deletion and not to CD19 hemizygosity (supplemental Figure 1B).
KAP1-deficient mice display reduced numbers of mature B cells. (A) Bone marrow (top), spleen (middle), and PE (bottom) cells from 8- to 12-week-old CD19-Cre/KAP1flox (KO) and littermate wt/KAP1flox control mice (ctrl) were counted and analyzed by flow cytometry. Left panels, total number of B cells; middle panels, representative flow cytometric analysis; right panels, average and SD of the percentage of the indicated populations, n ≥ 15. (B) CD19-Cre/YFPflox/Kap1flox (KO) and CD19-Cre/YFPflox/Kap1floxhet (ctrl) mice analyzed by flow cytometry as in panel A. Percentages of YFP+ and YFP− cells in the depicted populations are given as average and SEM (n = 5). (C) Chimeric mice obtained by transplantation of 50% CD45.2+CD19-Cre/YFPflox/Kap1flox plus 50% CD45.1+ wild-type (KO 50/50; n = 8), 50% CD45.2+CD19-Cre/YFPflox/KAP1wt plus 50% CD45.1+ wild-type (ctrl 50/50; n = 8), 100% CD45.2+CD19-Cre/YFP-flox/Kap1flox (KO 100; n = 3) and 100% CD45.2+CD19-Cre/YFPflox/Kap1wt (ctrl 100; n = 4) lineage-depleted cells were analyzed by flow cytometry 6-10 weeks after injection. Only CD45.2+ deleted (YFP+) cell frequencies are shown. For CD45.2+ nondeleted (YFP−) frequencies, see supplemental Figure 1. Subpopulations were analyzed as in panel A. Average and SD are shown. P values are for the indicated populations comparing KO50/50 with ctrl 50/50. Pre indicates Pre-B cell progenitors; Imm, immature B-cell progenitors; Mat, mature B-cell progenitors; T1, transitional 1 progenitors; T2-FP, transitional 2 follicular progenitors; and T2-MZP, transitional 2 MZ progenitors. Also see supplemental Figure 1. P values analyzed by Mann-Whitney test.
KAP1-deficient mice display reduced numbers of mature B cells. (A) Bone marrow (top), spleen (middle), and PE (bottom) cells from 8- to 12-week-old CD19-Cre/KAP1flox (KO) and littermate wt/KAP1flox control mice (ctrl) were counted and analyzed by flow cytometry. Left panels, total number of B cells; middle panels, representative flow cytometric analysis; right panels, average and SD of the percentage of the indicated populations, n ≥ 15. (B) CD19-Cre/YFPflox/Kap1flox (KO) and CD19-Cre/YFPflox/Kap1floxhet (ctrl) mice analyzed by flow cytometry as in panel A. Percentages of YFP+ and YFP− cells in the depicted populations are given as average and SEM (n = 5). (C) Chimeric mice obtained by transplantation of 50% CD45.2+CD19-Cre/YFPflox/Kap1flox plus 50% CD45.1+ wild-type (KO 50/50; n = 8), 50% CD45.2+CD19-Cre/YFPflox/KAP1wt plus 50% CD45.1+ wild-type (ctrl 50/50; n = 8), 100% CD45.2+CD19-Cre/YFP-flox/Kap1flox (KO 100; n = 3) and 100% CD45.2+CD19-Cre/YFPflox/Kap1wt (ctrl 100; n = 4) lineage-depleted cells were analyzed by flow cytometry 6-10 weeks after injection. Only CD45.2+ deleted (YFP+) cell frequencies are shown. For CD45.2+ nondeleted (YFP−) frequencies, see supplemental Figure 1. Subpopulations were analyzed as in panel A. Average and SD are shown. P values are for the indicated populations comparing KO50/50 with ctrl 50/50. Pre indicates Pre-B cell progenitors; Imm, immature B-cell progenitors; Mat, mature B-cell progenitors; T1, transitional 1 progenitors; T2-FP, transitional 2 follicular progenitors; and T2-MZP, transitional 2 MZ progenitors. Also see supplemental Figure 1. P values analyzed by Mann-Whitney test.
Because Kap1 deletion in total CD19+ cells was not complete (see supplemental Figure 1A), we investigated whether Kap1-excised cells were equally represented at all differentiation stages. We sorted T1, T2-FP, T2-MZ, FO, and MZ cell populations from the spleens of mutant mice and analyzed their genomic DNA by semiquantitative PCR. We found that all of the populations were efficiently excised (approximately 90%) except for the MZ, in which the excision efficiency was less than 60%, indicating a selective disadvantage of the KAP1-deficient cells in this subpopulation (supplemental Figure 1C). These data were further supported by the analysis of the mouse strain generated by breeding Kap1-deleted mice with stopfloxYFP animals, in which Cre induction results in yellow fluorescent protein (YFP) expression.23 After verifying that there was a direct correlation between Kap1 excision and YFP fluorescence (supplemental Figure 1D), we compared the frequency of YFP+ cells in the different B-cell subpopulations in KAP1-deficient and KAP1-competent mice. The results not only confirmed that B-lymphoid Kap1-KO mice harbored a reduced frequency of spleen MZ and FO and of B1 cells in PECs, but also that Kap1-deleted cells were significantly underrepresented among spleen MZ and B1 cells (Figure 1B). These data indicate that KAP1 is required for the efficient production and/or maintenance of a mature B-cell compartment.
To consolidate our findings, we generated BM chimeric mice by transplanting CD45.2+ BM-derived lineage-depleted hematopoietic progenitor cells purified from CD19-Cre/Kap1flox/stopfloxYFP–deficient or CD19-Cre/Kap1wt/stopfloxYFP control donor mice (mixed or not in equal proportion with CD45.1+ wild-type lineage-depleted hematopoietic progenitor cells) in CD45.1+ mice. Flow cytometric analysis at 6-10 weeks after reconstitution revealed an exacerbation of the phenotype observed in the non-chimeric-deficient mice, with a significant decrease in the frequency of Kap1-deleted CD45.2+ T2-FP, T2-MZ, MZ, and FO cells in the spleen, as well as B1a and B1b cells in the PECs (although reconstitution from donor-derived cells was minimal in this latter compartment, as expected; Figure 1C). Furthermore, we observed an accumulation of Kap1-deleted CD45.2+ pre-B cells in the BM of chimeric mice engrafted by 50% deficient and 50% wild-type cells (KO 50/50), compared with controls (ctrl 50/50). The same trend was observed in the CD45.2+ deleted cells in chimeric mice engrafted by 100% deficient (KO 100) cells, but not in the CD45.2+ nondeleted cells, compared with the respective controls (Figure 1C and supplemental Figure 1E). Therefore, early B-cell differentiation was significantly affected by KAP1 removal in mice harboring chimeric BM. Our phenotypic analysis indicates that KAP1 regulates the early and late differentiation of both conventional and nonconventional B cells.
KAP1-deficient mice display defects in Ab production
Even though the presence of Kap1-deleted mature B cells indicated that BCR rearrangement occurred in the absence of KAP1, we verified by PCR that BCR polyclonality was not compromised in this setting. We did not observe any difference in the distribution of the rearranged bands comparing splenocytes harvested from deficient and control mice. This indicated that KAP1 deletion did not induce a major skew in the usage of the variable Ig regions (supplemental Figure 2A).
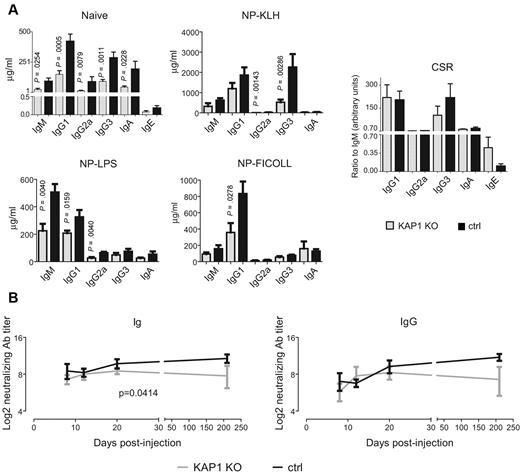
We next assessed the functionality of KAP1-deficient B cells. Serum levels of Abs of all isotypes were significantly reduced in KO compared with control naive mice (Figure 2A), suggesting a defect in steady-state Ab production. We then immunized mice by NP-KLH, NP-LPS, and NP-Ficoll injections to test T cell–dependent, T cell–independent type 1, and T cell–independent type 2 Ab responses, respectively, and measured serum Ab titers at day 21 after injection. The total Ab levels were lower in B-lymphoid Kap1-deleted mice than in control mice for all isotypes and all immunization protocols, but KO animals could mount both T cell–dependent and T cell–independent NP-specific Ab responses (Figure 2A and supplemental Figure 2B). Furthermore, CSR efficiently proceeded in mice immunized in the presence of aluminum adjuvant, albeit with a bias toward IgE in the absence of KAP1 (Figure 2A). In addition, B-lymphoid Kap1-deleted mice produced normal levels of neutralizing Abs early after immunization with either UV-treated vesicular stomatitis virus (VSV; not shown) or with recombinant adenoviral vector particles expressing the VSV envelope glycoprotein (rAD/VSV-G). However, neutralizing Ab levels dropped significantly faster in Kap1-KO mice than in control mice, indicating that KAP1 contributes to the maintenance of Ab memory (Figure 2B).
Defective immune responses in B-lymphoid KAP1-deficient mice. (A) ELISA detecting total Ig was performed on CD19-Cre/Kap1flox (KAP1 KO) and littermate wt/Kap1flox control (ctrl) mouse sera either at steady-state (top left panel, naive) or 21 days after immunization with the indicated agents (middle and bottom panels). Right panel, CSR calculated as the ratio between the amount of indicated isotype and IgM in mice injected with NP-KLH or NP-LPS and alum. Average and SEM are shown (n ≥ 4). P values are by 1-tailed Mann-Whitney test. (B) Serum titers of neutralizing total Ig and IgG from CD19-Cre/Kap1flox (KAP1 KO) and littermate wt/Kap1flox control (ctrl) mice injected with rAD/VSV-G. Sera were analyzed at days 8, 12, 20, and 210. Mean and SEM are shown (n = 4). P values are by 2-way ANOVA test; only significant P values are depicted. Also see supplemental Figure 2.
Defective immune responses in B-lymphoid KAP1-deficient mice. (A) ELISA detecting total Ig was performed on CD19-Cre/Kap1flox (KAP1 KO) and littermate wt/Kap1flox control (ctrl) mouse sera either at steady-state (top left panel, naive) or 21 days after immunization with the indicated agents (middle and bottom panels). Right panel, CSR calculated as the ratio between the amount of indicated isotype and IgM in mice injected with NP-KLH or NP-LPS and alum. Average and SEM are shown (n ≥ 4). P values are by 1-tailed Mann-Whitney test. (B) Serum titers of neutralizing total Ig and IgG from CD19-Cre/Kap1flox (KAP1 KO) and littermate wt/Kap1flox control (ctrl) mice injected with rAD/VSV-G. Sera were analyzed at days 8, 12, 20, and 210. Mean and SEM are shown (n = 4). P values are by 2-way ANOVA test; only significant P values are depicted. Also see supplemental Figure 2.
Transcriptional dysregulation in Kap1-deleted B cells
To identify the genes targeted by KAP1 in B cells, we performed microarray gene-expression analyses on the cell populations most affected phenotypically by the deletion of this transcriptional regulator. We extracted RNA from T2-FP and FO lymphocytes purified from the spleens of CD19-Cre/Kap1flox mice and littermate controls and verified by quantitative PCR (Q-PCR) that Kap1 depletion was approximately 90% in both cell populations from mutant mice (supplemental Figure 3A). We found 207 and 195 genes, respectively, dysregulated in T2-FP and FO Kap1-deleted cells compared with controls (P < .01), with approximately equal proportions of up- and down-regulated genes (Figure 3A; for the complete list of dysregulated genes, see supplemental Table 1). Functional annotation using the DAVID bioinformatics resource25,26 indicated that genes related to RNA processing/metabolic processes and immune system development were highly enriched in dysregulated genes in T2-FP–deficient progenitors, whereas in mature FO-deficient cells, the most represented classes were linked to metabolic processes and response to various forms of stress, including DNA damage (Figure 3B). These data further support a role of KAP1 in both the differentiation of progenitor B lymphocytes and the homeostasis of mature B lymphocytes.
Transcriptome analyses of B-lymphoid KAP1-deficient splenocytes. (A) Number of genes dysregulated in T2-FP and FO Kap1-deleted cells compared with controls. Up arrow indicates the number of up-regulated genes; down arrow, number of down-regulated genes. (B) DAVID bioinformatics database analysis of dysregulated genes. Gene Ontology biological process classes of genes enriched in the dysregulated gene lists for T2-FP (top panel) and FO (bottom panel) KAP1-deficient cells. The fold enrichment and P values are shown. DDR indicates DNA-damage response; intrac, intracellular; metab, metabolic; proc., process; resp, response; biosynth, biosynthetic; rns, ribonucleoside; ns, nucleoside; and 3P, triphosphate. (C) The fold dysregulation of the depicted genes as assessed by array, Q-PCR on RNA extracted from C57/bl6-129sv CD19-Cre/Kap1flox–deficient and littermate wt/Kap1flox controls (Q-PCR mix) and Q-PCR on RNA extracted from C57/bl6 CD19-Cre/Kap1flox–deficient and CD19-Cre/Kap1wt controls (Q-PCR C57/bl6). Averages ± SD are shown (n = 3). Top panel shows the T2-FP population; bottom panel, FO population. p/a indicates present in Kap1-deleted and absent in control samples. Also see supplemental Figure 3 and supplemental Table 1.
Transcriptome analyses of B-lymphoid KAP1-deficient splenocytes. (A) Number of genes dysregulated in T2-FP and FO Kap1-deleted cells compared with controls. Up arrow indicates the number of up-regulated genes; down arrow, number of down-regulated genes. (B) DAVID bioinformatics database analysis of dysregulated genes. Gene Ontology biological process classes of genes enriched in the dysregulated gene lists for T2-FP (top panel) and FO (bottom panel) KAP1-deficient cells. The fold enrichment and P values are shown. DDR indicates DNA-damage response; intrac, intracellular; metab, metabolic; proc., process; resp, response; biosynth, biosynthetic; rns, ribonucleoside; ns, nucleoside; and 3P, triphosphate. (C) The fold dysregulation of the depicted genes as assessed by array, Q-PCR on RNA extracted from C57/bl6-129sv CD19-Cre/Kap1flox–deficient and littermate wt/Kap1flox controls (Q-PCR mix) and Q-PCR on RNA extracted from C57/bl6 CD19-Cre/Kap1flox–deficient and CD19-Cre/Kap1wt controls (Q-PCR C57/bl6). Averages ± SD are shown (n = 3). Top panel shows the T2-FP population; bottom panel, FO population. p/a indicates present in Kap1-deleted and absent in control samples. Also see supplemental Figure 3 and supplemental Table 1.
We used Q-PCR to quantify more precisely a selected set of dysregulated transcripts in wild-type and Kap1-KO mice from both mixed and C57/bl6 backgrounds (Figure 3C). In both T2-FP and FO cells, the RNAs most highly up-regulated corresponded to glutathione S-transferases of the θ class. Whereas it remains to be determined whether these detoxifying enzymes fulfill specific functions in lymphocytes, several of the other transcripts deregulated by KAP1 removal pertained to B-cell signaling. PTEN (inositol phosphatase and tensin homolog), an enzymatic counter-regulator of the PI3K pathway, was found to be up-regulated in FO cells by both Q-PCR and Western blot (Figure 3C and supplemental Figure 3B). Consistent with this result, protein levels of FoxO1, a factor negatively regulated by PI3K, were increased in Kap1-deleted cells (supplemental Figure 3B). Similarly, levels of lymphoid-specific BTB-kelch protein KLHL6 RNA were increased in both T2-FP and FO Kap1-deleted cells, which is important considering the previously described involvement of this factor in BCR signaling and germinal center formation.27 KAP1 removal also induced an up-regulation of genes encoding for some immune receptors (ie, Ly6k, Gpr34, Slamf9, and Il9r) and transcription factors (ie, Zfp52 and Klf9) and for the mismatch-repair-mut-l-homolog-6 (Msh6) protein. MSH6 has been implicated in damage recognition, regulation of cell proliferation and apoptosis, and modulation of Ab diversification.28,29 Other genes encoding proteins involved in the cell cycle and apoptosis, such as CDC25B and TRP53 in T2-FP cells and cyclins D2 and D3 in FO cells, and in cytoskeleton rearrangement and cell migration, such as the chemokine receptors CXCR5 and CCR6 and the cytoskeleton remodeler PACSIN1 in FO cells and the actin-related protein ACTR3 in T2-FP, were also up-regulated in Kap1-KO cells. This links KAP1-mediated regulation with the migration and proliferation pathways in both transitional progenitors and mature B cells. Endogenous retro-elements were not deregulated in KAP1-deficient cells (data not shown), as expected from their irreversible silencing through DNA methylation after the early embryonic period.18
KAP1 binding and chromatin modifications in B cells
To discriminate between primary KAP1 targets and secondarily deregulated genes, we performed ChIP studies of B-enriched splenocytes extracted from wild-type and littermate Kap1-deleted mice using an Ab that recognizes the KAP1 RBCC domain.11 We subjected the KAP1-specific immunoprecipitates to deep sequencing and, after mapping the reads, used the ChIP-Seq Analysis Server (http://ccg.vital-it.ch/chipseq/) to identify significant peaks. We obtained 32 811 discrete peaks in the wild-type sample and 5886 in its KAP1-depleted counterpart. By intersecting the 2 lists, we found that approximately 75% (24 535) of the wild-type peaks had no overlapping peak in the Kap1-deleted sample (defined as a peak within a ± 1kb window from the interrogated peak). Reciprocally, more than 80% (4990) of the peaks mapped in the Kap1-deleted sample had an overlapping peak in the wild-type, probably originating largely from the background of Kap1-undeleted cells (Figure 4A). This strongly suggests that the majority of peaks identified in the wild-type sample represents bona fide KAP1 binding sites. We performed a ChIP/Seq-mediated census of H3K9me3-bearing regions in the same 2 samples, as KAP1 action typically leads to the deposition of this mark in vitro.10,12 We found 1784 and 1355 such regions in B-enriched splenocytes extracted from wild-type and littermate Kap1-deleted mice, respectively. Among these, half of the H3K9me3-enriched regions found in the wild-type were also present in the Kap1-deleted samples, whereas almost two-thirds of the H3K9me3 peaks detected in the KO sample overlapped with peaks recorded in the wild-type (supplemental Figure 4A).
KAP1 binding sites and chromatin modifications in B cells. Chromatin from wild-type and KAP1-deficient B splenocytes was immunoprecipitated with a KAP1- or H3K9me3-specific Ab and captured DNA was sequenced. (A) KAP1 binding sites identified in wild-type (left) and KAP1-deficient (KO; right) B splenocytes. Overlapping: number of wild-type (left) or KO (right) sites overlapping with at least 1 site identified in the KO (left) or wild-type (right) sample. The window set for the overlapping was ± 1 kb. (B) Stratification of the indicated features around KAP1 binding sites (set at 0), P values and correlation coefficient (R) are by Spearman test. (C) UCSC genes were sorted on the basis of expression as assessed in the microarray wild-type FO samples (supplemental Table 1) and distance to the nearest KAP1 H3K9me3 peak was evaluated. Absent indicates genes with expression signal < 30; Low-Mid, signal between 31 and 1000; and Hi, signal between 1001 and 45 000. Boxplots of boot-strapped values are shown. P values by Kruskal-Wallis test. Dunn posttest by pairs are: absent versus low-mid, P < .05; absent versus high, P < .05; and low-mid versus high, P > .05. Also see supplemental Figure 4.
KAP1 binding sites and chromatin modifications in B cells. Chromatin from wild-type and KAP1-deficient B splenocytes was immunoprecipitated with a KAP1- or H3K9me3-specific Ab and captured DNA was sequenced. (A) KAP1 binding sites identified in wild-type (left) and KAP1-deficient (KO; right) B splenocytes. Overlapping: number of wild-type (left) or KO (right) sites overlapping with at least 1 site identified in the KO (left) or wild-type (right) sample. The window set for the overlapping was ± 1 kb. (B) Stratification of the indicated features around KAP1 binding sites (set at 0), P values and correlation coefficient (R) are by Spearman test. (C) UCSC genes were sorted on the basis of expression as assessed in the microarray wild-type FO samples (supplemental Table 1) and distance to the nearest KAP1 H3K9me3 peak was evaluated. Absent indicates genes with expression signal < 30; Low-Mid, signal between 31 and 1000; and Hi, signal between 1001 and 45 000. Boxplots of boot-strapped values are shown. P values by Kruskal-Wallis test. Dunn posttest by pairs are: absent versus low-mid, P < .05; absent versus high, P < .05; and low-mid versus high, P > .05. Also see supplemental Figure 4.
We then performed correlation studies to better characterize the KAP1-bound genomic regions. We examined the distribution around KAP1 binding sites of different histone marks and transcription factors using datasets generated in B splenocytes by others and by us.21 KAP1-bound regions were significantly enriched in H3K9me3 and depleted in H3K4me3, a mark typically found at active and/or poised promoters.30 In addition, KAP1 peaks lacked regions bearing the H3K4me1 mark and PU.1 binding sites, which together characterize cell-specific enhancer-like loci21 (Figure 4B). Therefore, in B splenocytes, KAP1 is associated with genomic loci adorned with repressive modifications, whereas it is excluded from active promoter/enhancer elements. To be more stringent and consider only the heterochromatin-associated role of KAP1, we zoomed onto KAP1 peaks that coincided with H3k9me3-enriched regions (< 1kb distance), which represented approximately 30% of the total KAP1 binding sites (supplemental Figure 4B). We first calculated the distance from all genes to the nearest KAP1/H3K9me3 peak and crossed these data with the results of our transcriptional analyses in FO cells, because these account for approximately 80% of total B splenocytes. Although there was a slight trend for genes up-regulated in Kap1-KO cells to be closer to and down-regulated genes to be farther from KAP1 peaks than average, the differences between median distances were not significant (supplemental Figure 4C), indicating that proximity is not the only factor involved in KAP1-mediated regulation. We then investigated the correlation between absolute gene expression and distance to KAP1/H3K9me3 peaks and found that expressed genes (according to our microarray data) were significantly closer to KAP1/H3K9me3 peaks than nonexpressed genes (Figure 4C). This observation might seem contradictory for a repressive molecule such as KAP1; however, we think that it only indicates that KAP1 is not associated in vivo with constitutive silent chromatin, but rather with regions in which expression needs to be finely regulated. As an alternative explanation, KAP1 might show a preference for gene-rich regions, which, at least in the human genome, have been demonstrated to contain most of the expressed genes.31 We then mapped the closest gene to each KAP1/H3K9me3 peak and used the list of closest genes to peaks (≤ 20 kb) to interrogate the DAVID bioinformatics resource.25,26 We found cell transcription–related, RNA processing–related, and cytokine response Gene Ontology biological processes classes to be significantly enriched, suggesting a direct KAP1-mediated regulation of these genes (supplemental Figure 4D). Interestingly, when we looked at Simple Modular Architecture Research Tool (SMART) protein domain classes, we found the KRAB-domain class to be the most enriched in the list of closest genes to KAP1/H3K9me3 peaks (supplemental Figure 4D). This is consistent with the previous observation that, in some human cell lines, KAP1 binds to and, in some cases, controls the expression of KRAB-ZFP genes.32-35
When we examined specific genes up-regulated in Kap1 KO FO or T2-FP cells (see Figure 3), we found 2 KAP1 sites within the Pten gene, one in an intron and one in the 3′ untranslated region. Furthermore, we observed that the distal KAP1 Pten peak coincided with a high H3K9me3 signal in wild-type cells, which strongly decreased in KO cells (Figure 5A). KAP1 and H3K9me3 peaks were also detected near the Msh6 and Slamf9 genes (supplemental Figure 5) and ChIP/Q-PCR with specific primers confirmed significant KAP1 and H3K9me3 enrichment at all of these sites (Figure 5B-C). Finally, we observed an enrichment of H3K9me3 and a depletion of the activation mark histone 3 acetylation (H3Ac) in wild-type compared with Kap1-deleted B splenocytes at the promoters of these genes (Figure 5D). These results confirm that all of these genes are direct targets of KAP1.
KAP1-mediated control of genes dysregulated in KAP1-deficient B cells. (A) Pten genomic locus with KAP1 and H3K9me3 ChIP/seq signal for Kap1 wild-type and KO B splenocytes. Chromosome and relative position (arrowhead), scale, UCSC-based transcripts (most abundant in black, least abundant in gray), CpG islands (suggestive of promoter regions), and position of Q-PCR primers used for validation in panels B, C, and D are depicted here as a1, a2, and b, respectively. Arrow indicates sense of transcription. (B-D) Chromatin from wild-type and KO B splenocytes was immunoprecipitated with KAP1-specific Abs (B), H3K9me3-specific Abs (C-D black bars), or H3Ac-specific Abs (D gray bars). Q-PCR in panels B, C, and D was performed with primers amplifying the region depicted in panel A and supplemental Figure 4 as a and b, respectively. Enrichment of immunoprecipitated samples is expressed as total input fraction (fold change over ctrl; C) normalized on the KO signal (fold change over KO; B,D). Average of the fold change in the control gene (GAPDH) was set at 1 (gray line). Averages plus SEM are shown (n = 4). *P < .05 compared with control gene signal by 1-tailed Mann-Whitney test. Also see supplemental Figure 5.
KAP1-mediated control of genes dysregulated in KAP1-deficient B cells. (A) Pten genomic locus with KAP1 and H3K9me3 ChIP/seq signal for Kap1 wild-type and KO B splenocytes. Chromosome and relative position (arrowhead), scale, UCSC-based transcripts (most abundant in black, least abundant in gray), CpG islands (suggestive of promoter regions), and position of Q-PCR primers used for validation in panels B, C, and D are depicted here as a1, a2, and b, respectively. Arrow indicates sense of transcription. (B-D) Chromatin from wild-type and KO B splenocytes was immunoprecipitated with KAP1-specific Abs (B), H3K9me3-specific Abs (C-D black bars), or H3Ac-specific Abs (D gray bars). Q-PCR in panels B, C, and D was performed with primers amplifying the region depicted in panel A and supplemental Figure 4 as a and b, respectively. Enrichment of immunoprecipitated samples is expressed as total input fraction (fold change over ctrl; C) normalized on the KO signal (fold change over KO; B,D). Average of the fold change in the control gene (GAPDH) was set at 1 (gray line). Averages plus SEM are shown (n = 4). *P < .05 compared with control gene signal by 1-tailed Mann-Whitney test. Also see supplemental Figure 5.
A subset of KRAB-ZFPs is differentially expressed in B-lymphoid cells
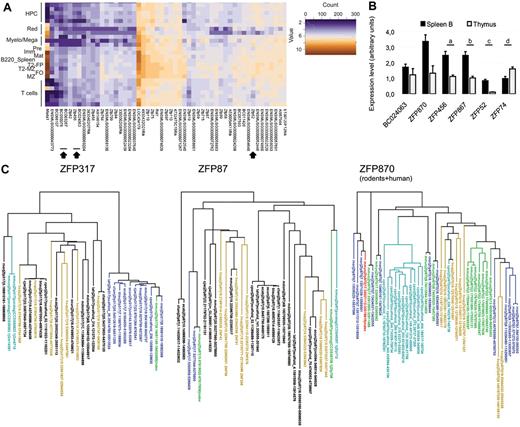
We also investigated which members of the KRAB-ZFP family are expressed in B-lymphoid cells to account for KAP1 recruitment to at least some of its genomic targets. We isolated RNA from a large series of cells purified by FACS from the BM, spleens, and thymi of wild-type mice, representing some 26 stage/lineage-specific steps of lymphohematopoietic differentiation. We then quantified transcripts from 304 murine Krab-Zfps and 25 control genes using a custom probe set (282 probes) for the NanoString nCounter platform36 (for a complete list of all tested hematopoietic populations and staining strategies used for their isolation see supplemental Methods). We and others have validated this approach previously by correlating its results with those of Q-PCR, microarray, and RNA-seq analyses37,38 (data not shown). Sixty-eight and 89 of these probes yielded uniformly strong or weak signals, respectively, in all tested cell types (not shown). However, 54 and 35 probes identified KRAB-ZFPs or clusters that were expressed at higher or lower levels, respectively, in B cells than in all other lineages (Figure 6A and supplemental Figure 6A). Q-PCR confirmed the B cell–specific pattern of expression of all of the differentially expressed genes tested (Figure 6B).
KRAB-ZFP expression in B cells. (A) Heat map of KRAB-ZFP (and control genes) that were significantly more expressed in B cells than in other hematopoietic lineages as assessed by NanoString nCounter direct RNA quantification. For the staining strategy used to sort the different populations, see supplemental Methods. HPC indicates hematopoietic progenitor cells; red, erythrocyte lineage; and Myelo/Mega, myeloid/megakaryocyte. For a description of the B-cell population, see Figure 1 legend. Arrowheads indicate KRAB-ZFP analyzed in panel B. (B) Q-PCR analysis performed on cDNA from thymus and B-enriched splenocytes (spleen B), using primers specific for the indicated KRAB-ZFP (indicated by arrowheads in panel A and supplemental Figure 6A). P = .0046; b, P = .0022; c, P = .0022; and d, P = .041 by Mann-Whitney test. Averages plus SEM are shown (n = 6). (C) Phylogenetic trees of the depicted KRAB-ZFP based on ZF exon sequence alignment. Each protein is defined by the genome: mouse, mmus; human, hsap; macaque, mmul; marmoset, cjac; cow, btau; dog, cfam; horse, ecab; rat, rnor; guinea pig; cpor; rabbit, ocun; elephant, lafr; and opossum, mdom. Query protein is depicted as index, in green in the left and middle panels and in red in the right panel. Green indicates mouse paralogs present in the list of differentially expressed ZFP in B cells (supplemental Figure 4); blue, 1:1 orthologs; aqua, closest rodent paralogs; and mustard, closest human genome matches. In the right panel, only rodents and human hits are shown (rodents + human). Also see supplemental Figure 6.
KRAB-ZFP expression in B cells. (A) Heat map of KRAB-ZFP (and control genes) that were significantly more expressed in B cells than in other hematopoietic lineages as assessed by NanoString nCounter direct RNA quantification. For the staining strategy used to sort the different populations, see supplemental Methods. HPC indicates hematopoietic progenitor cells; red, erythrocyte lineage; and Myelo/Mega, myeloid/megakaryocyte. For a description of the B-cell population, see Figure 1 legend. Arrowheads indicate KRAB-ZFP analyzed in panel B. (B) Q-PCR analysis performed on cDNA from thymus and B-enriched splenocytes (spleen B), using primers specific for the indicated KRAB-ZFP (indicated by arrowheads in panel A and supplemental Figure 6A). P = .0046; b, P = .0022; c, P = .0022; and d, P = .041 by Mann-Whitney test. Averages plus SEM are shown (n = 6). (C) Phylogenetic trees of the depicted KRAB-ZFP based on ZF exon sequence alignment. Each protein is defined by the genome: mouse, mmus; human, hsap; macaque, mmul; marmoset, cjac; cow, btau; dog, cfam; horse, ecab; rat, rnor; guinea pig; cpor; rabbit, ocun; elephant, lafr; and opossum, mdom. Query protein is depicted as index, in green in the left and middle panels and in red in the right panel. Green indicates mouse paralogs present in the list of differentially expressed ZFP in B cells (supplemental Figure 4); blue, 1:1 orthologs; aqua, closest rodent paralogs; and mustard, closest human genome matches. In the right panel, only rodents and human hits are shown (rodents + human). Also see supplemental Figure 6.
Based on alignments of their ZF exon sequences (supplemental Figure 6B), only 8 of the 49 murine KRAB-ZFPs most highly expressed in B cells have a human ortholog, which is consistent with the marked evolutionary divergence of rodents and primates in this gene family.39 Nevertheless, one of these conserved B cell–expressed murine KRAB-ZFP is ZFP317, the human ortholog of which has been proposed to play a role in lymphocyte proliferation40 (Figure 6C). Of the majority of rodent-specific KRAB-ZFPs in our list, some displayed the signs of a significant expansion in this clade. Many paralogs could be found for several of them, including ZFP870, suggesting that they were subjected to positive selection and might have related functions; one of them, ZFP87, has paralogs in the human genome, namely ZNF91 and ZNF43, which are highly expressed in lymphoid cells.41
Discussion
Recent studies have proposed that epigenetic regulators control the development and homeostasis of B cells, notably by determining the accessibility of specific genomic loci to transcription factors. In the present study, we investigated the role of KAP1, the universal essential cofactor of the large family of KRAB-ZFPs, in the differentiation and function of B lymphocytes.
We inactivated KRAB-ZFP/KAP1-mediated regulation by conditional deletion of the Kap1 locus at the pro-B stage of B-cell differentiation. Although the deletion was not complete, mutant mice displayed a clear reduction in mature FO and MZ B splenocytes and in peritoneal B1 cells, with a significant overgrowth of residual wild-type over Kap1-deleted cells in the MZ and B1 populations. We also observed an accumulation of T2-FP progenitor cells in the KO mice, and an even more pronounced accumulation of BM early B progenitors when Kap1-deleted cells were placed in competition with wild-type cells. Furthermore, KAP1-defective mice harbored abnormally low levels of steady-state Abs of all isotypes. In mice kept under pathogen-free conditions, natural Abs (largely IgM, IgA, and IgG3, made in the absence of specific antigens by B1 cells) and Abs against commensal bacteria and food antigens constitute so-called steady-state Abs.42 The observed reduced levels of these Abs can therefore be explained by the lower numbers of B1 cells and/or to a functional defect of these cells in Kap1-deleted mice. Although the latter responded to challenge with antigenic peptides, they displayed faster rates of decay of neutralizing Abs after viral immunization. Whether this results from a defect in long-lived reactions in germinal centers or from a failure to maintain plasma cells in the BM remains to be defined, but these data indicate that KAP1 contributes to long-term Ab memory. We did not investigate whether KAP1 affects other B-cell functions such as cytokine production and antigen presentation. Nevertheless, we observed that, whereas Kap1-depleted mice performed CSR at normal rates, they exhibited a bias toward IgE on immunization. Because IgEs are the principal mediators of allergic reactions, this result suggests that KAP1 and perhaps specific KRAB-ZFPs may be involved in controlling allergies. Our finding of normal rates of CSR in the absence of KAP1 is in apparent conflict with the recent proposal that KAP1 is needed for CSR.43 However, this other work was performed in vitro, and it could be that the 2-fold decrease in CSR efficiency detected in this other setting is fully compensated for in vivo.
Gene ontology analyses of the transcripts dysregulated in KAP1-deficient T2-FP cells revealed a significant enrichment in genes involved in immune system development. This is consistent with the observed phenotype and further supports a role for KAP1 in B-lymphoid differentiation. The DNA mismatch repair (MMR) protein MSH6 was up-regulated in both T2-FP and FO cells. The MMR system is needed to maintain genomic stability, and components of the MMR regulate signal transducers are involved in cell-growth arrest and cell death.44 Kap1 deletion also led to dysregulation of factors involved in cytoskeletal rearrangement and migration in response to external stimuli transduced by the BCR and chemokine receptors, such as KLHL6, CXCR5, and SLAM9.27,45
Several observations suggest that the loss of KAP1-mediated repression of PTEN, an antagonist of PI3K, plays a prominent role in the phenotype of B-lymphoid Kap KO mice. First, PTEN transcripts were up-regulated in Kap1-deleted FO B lymphocytes and this correlated with an increase in FoxO1, a factor normally down-regulated by PI3K. Correspondingly, we detected 2 KAP1 binding sites in the Pten gene, and observed KAP1-dependent H3K9me3 at one of these sites and at the Pten promoter, indicating direct repression of this gene by KAP1. PI3K, through its role in secondary messenger production in response to BCR signaling, controls both early and peripheral B-cell development and homeostasis, a process modulated by PTEN.46 Furthermore, it was observed previously that loss of PTEN leads to an expansion of the MZ and B1 cell compartments and to increased autoantibody production.47 Finally, PI3K negatively regulates IgE production.48 Therefore, it is tempting to relate the decreased numbers of MZ and B1 cells, the lower levels of steady-state Abs, and the increased rates of IgE CSR noted in B lymphoid Kap1 KO mice to a loss of KAP1-mediated repression of Pten.
Our chromatin studies also detected sites of KAP1 binding and H3K9me3 deposition near the Msh6 and Slamf9 genes. As for Pten, the promoters of these genes became activated after KAP1 removal and this was accompanied by H3K9me3 depletion and H3Ac enrichment, correlating with the up-regulation of their transcripts in this setting. This strongly suggests that KAP1 controls directly the expression of these genes involved in the DNA-damage response and cytoskeleton/migration. This could also explain partly the defect in maintenance of long-term Ab memory noted in Kap1-deleted mice, because MSH6 has been shown to be involved in the control of germinal center homeostasis and because SLAM molecules have been shown to be needed for long-term humoral reactions.49,50 Finally, Kap1 deletion led to significant up-regulation of the serine protease inhibitor clade A3 (Serpina3) F-G genes and the glutathione S-transferase θ (Gstt) cluster. The functions of the products of these 2 gene clusters in the B-lymphoid lineage are undefined, and the results of our analyses warrant exploring further their role in B-cell development.
Genome-wide analysis of KAP1-bound regions revealed that they were enriched in the H3K9me3 repressive histone mark and depleted of modifications associated with active/poised B-specific cis-regulatory regions. To our knowledge, the present study is the first to characterize KAP1 binding sites in an in vivo adult tissue. It confirms the association of this molecule with facultative heterochromatic regions and strongly supports its role as an orchestrator of gene silencing.
KAP1 has no DNA-binding domain and its association with specific genomic loci is governed via other proteins, notably products of the Krab-Zfp gene family. KRAB-ZFPs constitute the largest group of transcriptional regulators encoded by the mammalian genome, and have been proposed to exhibit tissue-restricted modes of expression.7,39 By analyzing the expression of 300 of these, accounting for approximately 85% of the mouse KRAB-ZFPs according to our identification strategies, we determined that approximately one-third were highly expressed in most hematopoietic cells, one-third were globally low in all of these cells, and one-third were differentially expressed in the B lineage. Although drawing parallels between human and mouse KRAB-ZFPs might be misleading because of their divergent evolution,39 this pattern of expression is reminiscent of the one described in human lymphoid tissues.7 Interestingly, although most Krab-Zfp genes map to clusters generated by gene duplication of a few ancestors, we often found differential expression of the genes residing within a cluster, strongly suggesting functional divergence of duplicated genes and indicating tight regulation at the single gene level. When we tested for orthologs of B cell–enriched Krab-Zfp sequences in several tetrapod genomes, we found that approximately 80% of them were rodent specific, a proportion similar to that measured for the entire set of Krab-Zfp genes.39 The targets of most of the identified human orthologs of murine B cell–expressed KRAB-ZFPs are still un-defined, but ZNF317 has been proposed to regulate lymphocyte proliferation, and some isoforms are expressed exclusively in spleen, peripheral blood lymphocytes, and lungs.40
The results of the present study call for further studies aimed at identifying the KRAB-ZFPs interacting with specific KAP1 targets, such as Pten, and at determining the consequences of their overexpression or inactivation. Finally, because the rapidly evolving Krab-Zfp gene family exhibits significant polymorphism in humans, it will be interesting to investigate whether nucleotide substitutions in its B cell–expressed members might underlie interindividual differences in susceptibility to immune-related disorders such as infections, allergies, and autoimmune diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sonia Verp for technical help; Anna Groner, Helen Rowe, and Corinne Schär for fruitful discussions; Giovanna Ambrosini, Jacques Rougemont, Patrick Descombes, and the Genomics Platform of Frontiers in Genetics for help in high-throughput analyses; Fabio Aloisio for histological analysis; Jessica Dessimoz and the École Polytechnique Fédérale de Lausanne histology core facility, Miguel Garcia and the École Polytechnique Fédérale de Lausanne flow cytometry core facility, and Florian Kreppel and Stefan Kochanek from the University of Ulm for the rAD/VSV-G vector. Part of the computation was performed on the Vital-IT facility (www.vital-it.ch) of the Swiss Institute of Bioinformatics.
This work was supported by grants from the Swiss National Science Foundation and the European Research Council to D.T. P.G. was supported by the Associazione Italiana per la Ricerca sul Cancro, Milano, Italy.
Authorship
Contribution: F.R.S.d.S designed and performed the experiments, analyzed the data, and wrote the manuscript; J.M., A.C., K.B., P.G., D.P., and N.H. designed the experiments and provided the reagents; I.B., S.O., A.D., and M.F. performed the experiments; A.K. and J.H.T. analyzed the data; and D.T. designed the experiments, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Didier Trono, École Polytechnique Féderale de Lausanne, Science de la Vie-Doyen (EPFL SV-DO) Bâtiment SV, Station 19, CH 1015 Lausanne, Switzerland; e-mail: didier.trono@epfl.ch.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal