Abstract
Silent cerebral infarct (SCI) is the most common form of neurologic disease in children with sickle cell anemia (SCA). SCI is defined as abnormal magnetic resonance imaging (MRI) of the brain in the setting of a normal neurologic examination without a history or physical findings associated with an overt stroke. SCI occurs in 27% of this population before their sixth, and 37% by their 14th birthdays. In adults with SCA, the clinical history of SCI is poorly defined, although recent evidence suggests that they too may have ongoing risk of progressive injury. Risk factors for SCI include male sex, lower baseline hemoglobin concentration, higher baseline systolic blood pressure, and previous seizures. Specific morbidity associated with SCI includes a decrement in general intellectual abilities, poor academic achievement, progression to overt stroke, and progressive SCI. In addition, children with previous stroke continue to have both overt strokes and new SCI despite receiving regular blood transfusion therapy for secondary stroke prevention. Studies that only include overt stroke as a measure of CNS injury significantly underestimate the total cerebral injury burden in this population. In this review, we describe the epidemiology, natural history, morbidity, medical management, and potential therapeutic options for SCI in SCA.
Introduction
One of the most devastating medical complications of sickle cell anemia (SCA) is cerebral injury, which limits the full potential of the developing child and adult. The most common neurologic injury in SCA is silent cerebral infarct (SCI). Although there are few studies, SCI occurs in approximately one-quarter of children with SCA before their sixth birthday1 and approximately one-third before their 14th birthday.2 The first evidence that SCI had clinical significance was published in a cross-sectional computed tomography (CT) scan study of 25 patients. Five had cerebral infarcts but only 4 had a focal neurologic deficit (overt stroke).3 The investigators referred to the fifth patient as having a covert stroke,3 a term also used by others.4 The term SCI or silent stroke has gained widespread use to describe this condition because the Cooperative Study of Sickle Cell Disease (CSSCD) used standard clinical magnetic resonance imaging (MRI) to define the radiologic parameters.5 Subsequently, only a few studies of SCI have been completed. Until recently, the clinical importance of identifying SCI did not seem to justify the expense of MRI requiring sedation or anesthesia in preschool children, particularly in the absence of an effective intervention. Despite being the most common type of cerebral injury among children and adults with SCA, relatively little is known about the cause and optimal therapy of SCI. We will review the current literature on definition, epidemiology, natural history, clinical significance, medical management, and potential therapeutic options for the treatment of SCI in SCA.
Definition of SCI in children with SCA
The largest longitudinal cohort study to address the prevalence and incidence of SCI in children with SCA was the CSSCD, an observational study of children identified as infants and followed closely over the subsequent decade, which used MRI of the brain to detect abnormally increased T2-weighted signal intensity on multiple views (Figures 1 and 2) in children without current focal neurologic deficits as assessed by a hematologist. Previous neurologic history other than seizures, for example, coma, dizziness, ataxia, or transient focal weakness, was not explored. Hematologists may have discounted subtle neurologic findings that may have classified as abnormal and suggesting a prior overt stroke if reviewed by neurologists.6 The more precise definition used by Casella et al7 in The Silent Cerebral Infarct Trial (SIT Trial)7,8 included a MRI lesion measuring at least 3 mm in greatest linear dimension, visible in at least 2 planes of T2-weighted images (axial and coronal; Figure 2B-C). The SIT Trial definition excluded prior seizures and required no current focal neurologic deficit that could be explained by the anatomic location of the presumed SCI. Thus, individuals could have a focal neurologic examination, for example, compatible with a peripheral neuropathy, but would still meet the definition of SCI if the location of the infarct would not account for the neurologic deficit.
MRI in sickle cell disease. Coronal T1-weighted MRI (A,E) and axial T2-weighted MRI (B-D,F-H) in patients with homozygous SCA. (A-C) Normal MRI in a 19-year-old man with homozygous SCA. (D) Three years later, there is no change. (E-G) Silent infarction (arrows) in the frontal white matter and basal ganglia in a 15-year-old girl with cognitive problems affecting school performance but no acute neurologic presentation. (H) Three years later she has further infarcts with evidence of mild generalized atrophy and had a transient right hemiparesis as well as developing signs of a diplegia.
MRI in sickle cell disease. Coronal T1-weighted MRI (A,E) and axial T2-weighted MRI (B-D,F-H) in patients with homozygous SCA. (A-C) Normal MRI in a 19-year-old man with homozygous SCA. (D) Three years later, there is no change. (E-G) Silent infarction (arrows) in the frontal white matter and basal ganglia in a 15-year-old girl with cognitive problems affecting school performance but no acute neurologic presentation. (H) Three years later she has further infarcts with evidence of mild generalized atrophy and had a transient right hemiparesis as well as developing signs of a diplegia.
MRI in sickle cell disease. (A) Coronal T1-weighted MRI, (B) coronal T2-weighted MRI, and (C-H) axial T2-weighted MRI in patients with homozygous SCA. (A-C) Silent cerebral infarction (white arrows) in the parietal white matter in a 10-year-old girl with headache. (D) Three years later, there is progressive atrophy on MRI in the context of intermittent ataxia and squint. (E-H) Four cases associated with acute illness. (E) Silent cerebral infarction (black arrows) in the watershed regions between the anterior, middle, and posterior regions, including the deep white matter, in a patient who had previously had posterior reversible encephalopathy syndrome in the context of cyclosporine treatment for nephrotic syndrome. (F) Bilateral watershed infarction in a child who had seizures in the context of a facial infection. Motor examination was normal but his IQ was reduced by 30 points compared with premorbid testing. (G) Encephalomalacia after sagittal sinus thrombosis secondary to pneumococcal meningitis. (H) Occipital infarction after acute chest crisis. A homonymous visual field defect was detected after the infarct was noted on MRI.
MRI in sickle cell disease. (A) Coronal T1-weighted MRI, (B) coronal T2-weighted MRI, and (C-H) axial T2-weighted MRI in patients with homozygous SCA. (A-C) Silent cerebral infarction (white arrows) in the parietal white matter in a 10-year-old girl with headache. (D) Three years later, there is progressive atrophy on MRI in the context of intermittent ataxia and squint. (E-H) Four cases associated with acute illness. (E) Silent cerebral infarction (black arrows) in the watershed regions between the anterior, middle, and posterior regions, including the deep white matter, in a patient who had previously had posterior reversible encephalopathy syndrome in the context of cyclosporine treatment for nephrotic syndrome. (F) Bilateral watershed infarction in a child who had seizures in the context of a facial infection. Motor examination was normal but his IQ was reduced by 30 points compared with premorbid testing. (G) Encephalomalacia after sagittal sinus thrombosis secondary to pneumococcal meningitis. (H) Occipital infarction after acute chest crisis. A homonymous visual field defect was detected after the infarct was noted on MRI.
Alternate definition of SCI in adults with SCA
In the first systematic study of SCI in adults with SCA, Vichinsky et al used a definition of a minimum of 5 mm signal hyperintensity in the T2-weighted image,9 but to be included, lesions also had to show corresponding hypointensity on the T1-weighted image (Figures 1E, 2A). Normal adults typically accumulate T2 hyperintensities as they age, but children do not. This more restrictive definition of SCI and the distinction from encephalomalacia and atrophy (Figures 1H, 2D-H) parallels the descriptions used in general populations of asymptomatic elderly adults with SCI.10
Challenges with the definition of SCI
In addition to the varying definitions of SCI, lesion detection is dependent on the magnetic resonance (MR) technique (fluid attenuation inversion recovery [FLAIR] or diffusion-weighted imaging [DWI], see Figure 3, vs traditional T2-weighted images), the slice thickness,11 and the magnetic field strength. As further advances in imaging are made and medical centers transition from 1.5 Tesla to 3.0 Tesla magnets, more individuals with SCA will be detected with SCI, and quantitative techniques are likely to detect subtle abnormality in those without SCI on T2-weighted MRI.12,13 Use of the greater magnet strength will most likely have an impact on measures of incidence and prevalence of SCI, especially in longitudinal studies and CNS-related clinical trials. Thus, investigators must consider the anticipated change in the magnetic strength of the scanner over time when conducting a longitudinal study.
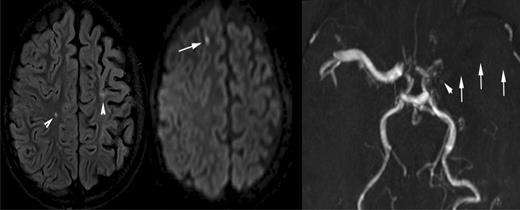
A 10-year-old boy with sickle cell disease and history of acute chest syndrome now presents with pain crisis. MRI of the brain was requested for episodic slurred speech. Axial FLAIR MR image (left) and DWI (middle) illustrate many of the manifestations of SCD in the brain. The arrowheads on the FLAIR image point out areas of old (silent) infarctions in the white matter of the centrum semiovale on the right and at the posterior aspect of the left superior frontal gyrus. The DWI shows an additional area of signal abnormality in the anterior aspect of the right superior frontal gyrus, representing a recent infarction. In addition, there is atrophy of the left cerebral hemisphere, which is seen in the setting of sickle cell–associated vasculopathy manifest by nonvisualization of the left middle cerebral artery by MRA (arrows on the right image). The MRA also shows subtle collaterals (moyamoya vessels) in the lenticulostriate distribution on the left (arrowhead).
A 10-year-old boy with sickle cell disease and history of acute chest syndrome now presents with pain crisis. MRI of the brain was requested for episodic slurred speech. Axial FLAIR MR image (left) and DWI (middle) illustrate many of the manifestations of SCD in the brain. The arrowheads on the FLAIR image point out areas of old (silent) infarctions in the white matter of the centrum semiovale on the right and at the posterior aspect of the left superior frontal gyrus. The DWI shows an additional area of signal abnormality in the anterior aspect of the right superior frontal gyrus, representing a recent infarction. In addition, there is atrophy of the left cerebral hemisphere, which is seen in the setting of sickle cell–associated vasculopathy manifest by nonvisualization of the left middle cerebral artery by MRA (arrows on the right image). The MRA also shows subtle collaterals (moyamoya vessels) in the lenticulostriate distribution on the left (arrowhead).
Strict guidelines for radiographic assessment of SCI require the ability to distinguish SCI from acute and chronic mimics of SCI (Figure 4). Because of the possibility of misclassification of a diagnosis of SCI when a new lesion is suspected, a dialogue between the neuroradiologist, neurologist, and hematologist should occur so both the clinical history and differential diagnoses can be carefully considered.
Differential diagnosis of silent infarction (images from patients without SCA). (A) Mimics of SCI: periventricular leukomalacia (PVL). A 20-month-old boy with cerebral palsy characterized by spastic diplegia. Axial FLAIR MR images illustrate classic findings of PVL. The image on the left is at the level of the centrum semiovale and demonstrates bilateral hyperintensities in the parietal lobe white matter. This appearance of the white matter overlaps with the presentation of SCI. The image on the right at the level of the basal ganglia illustrates dysmorphic lateral ventricles, thinning of the periventricular white matter and periventricular signal hyperintensity in a predominantly posterior distribution. Taken together, the images are consistent with the diagnosis of PVL in the setting of prematurity and cerebral palsy rather than SCI. (B) Terminal zones of myelination. A 2-year-old boy with a normal MRI of the brain. The Axial FLAIR MR image (left) shows ill-defined hyperintensity bilaterally in the deep white matter adjacent to the atria of the lateral ventricles (arrows). The T2-weighted image on the right illustrates that there are well-defined linear perivascular spaces (arrowheads) traversing the area of vague hyperintensity. This combination of findings is classic for the terminal zones of myelination, the last areas of the deep white matter to myelinate and displace free water. The terminal zones of myelination remain prominent through the second year of life and become progressively less conspicuous over time. They may be visible into the middle of the first decade of life. (C) Virchow-Robin spaces. A 12-year-old boy withT2-weighted (left) and Axial FLAIR (right) MR images with a normal MRI. The T2-weighted images reveal multiple punctuate white matter hyperintensities that suppresses on FLAIR indicating that the hyperintensities are indistinguishable from cerebrospinal fluid. The fluid attenuation feature of the FLAIR image helps to differentiate perivascular (Virchow-Robin) spaces from SCI. The arrows illustrate another feature of perivascular spaces which is that they appear linear when running within the slice. (D) Posterior reversible encephalopathy syndrome (PRES). A 14-year-old girl with altered mental status and seizures. Axial FLAIR MR images demonstrate hyperintensities bilaterally in the subcortical white matter and overlying cortex with predominant subcortical involvement. The distribution of the signal abnormalities is predominantly posterior and peripheral, a typical distribution for PRES. In contradistinction, SCIs favor the deep white matter of the frontal lobes. Nevertheless, clinical context is the key to differentiating PRES from SCIs. This is especially challenging in patients with SCD because they are prone to development of PRES and SCI. (E) Acute disseminated encephalomyelitis (ADEM). A 5-year-old boy with fever and headache. Axial FLAIR MR images demonstrate patchy, bilateral hyperintensities in the white matter of the centrum semiovale and corona radiata (arrows). Although the image on the left could be confused for SCI in the frontal border zone distribution, the middle and right image show subcortical and patchy hyperintensities that would be atypical in location, size, and lesion definition for SCI. The clinical information is the key to distinguishing lesions of ADEM from SCI.
Differential diagnosis of silent infarction (images from patients without SCA). (A) Mimics of SCI: periventricular leukomalacia (PVL). A 20-month-old boy with cerebral palsy characterized by spastic diplegia. Axial FLAIR MR images illustrate classic findings of PVL. The image on the left is at the level of the centrum semiovale and demonstrates bilateral hyperintensities in the parietal lobe white matter. This appearance of the white matter overlaps with the presentation of SCI. The image on the right at the level of the basal ganglia illustrates dysmorphic lateral ventricles, thinning of the periventricular white matter and periventricular signal hyperintensity in a predominantly posterior distribution. Taken together, the images are consistent with the diagnosis of PVL in the setting of prematurity and cerebral palsy rather than SCI. (B) Terminal zones of myelination. A 2-year-old boy with a normal MRI of the brain. The Axial FLAIR MR image (left) shows ill-defined hyperintensity bilaterally in the deep white matter adjacent to the atria of the lateral ventricles (arrows). The T2-weighted image on the right illustrates that there are well-defined linear perivascular spaces (arrowheads) traversing the area of vague hyperintensity. This combination of findings is classic for the terminal zones of myelination, the last areas of the deep white matter to myelinate and displace free water. The terminal zones of myelination remain prominent through the second year of life and become progressively less conspicuous over time. They may be visible into the middle of the first decade of life. (C) Virchow-Robin spaces. A 12-year-old boy withT2-weighted (left) and Axial FLAIR (right) MR images with a normal MRI. The T2-weighted images reveal multiple punctuate white matter hyperintensities that suppresses on FLAIR indicating that the hyperintensities are indistinguishable from cerebrospinal fluid. The fluid attenuation feature of the FLAIR image helps to differentiate perivascular (Virchow-Robin) spaces from SCI. The arrows illustrate another feature of perivascular spaces which is that they appear linear when running within the slice. (D) Posterior reversible encephalopathy syndrome (PRES). A 14-year-old girl with altered mental status and seizures. Axial FLAIR MR images demonstrate hyperintensities bilaterally in the subcortical white matter and overlying cortex with predominant subcortical involvement. The distribution of the signal abnormalities is predominantly posterior and peripheral, a typical distribution for PRES. In contradistinction, SCIs favor the deep white matter of the frontal lobes. Nevertheless, clinical context is the key to differentiating PRES from SCIs. This is especially challenging in patients with SCD because they are prone to development of PRES and SCI. (E) Acute disseminated encephalomyelitis (ADEM). A 5-year-old boy with fever and headache. Axial FLAIR MR images demonstrate patchy, bilateral hyperintensities in the white matter of the centrum semiovale and corona radiata (arrows). Although the image on the left could be confused for SCI in the frontal border zone distribution, the middle and right image show subcortical and patchy hyperintensities that would be atypical in location, size, and lesion definition for SCI. The clinical information is the key to distinguishing lesions of ADEM from SCI.
As is required by this definition, children with SCI do not have any obvious evidence of symptoms, other than perhaps academic or behavioral difficulties. Quite often, the presence of SCI is detected when the child presents with subtle neurologic findings prompting a brain MRI. The neuroradiologist may identify additional previous infarcts and distinguish an acute cerebral infarct from a previously unknown SCI (Figure 3A). A focal DWI hyperintensity indicates an ischemic injury within the last 8 to 10 days14 (Figure 3B), while the presence of brain atrophy suggests a chronic process.
Clinical significance of SCIs
As part of the protocol for the observational CSSCD cohort, the leadership group added serial surveillance MRI scans of the brain of children beginning at 6 years of age. A total of 266 children with SCA hemoglobin SS (HbSS) completed both a MRI of the brain and a battery of age-appropriate tests of cognitive function. The average age at initial scan was 8.3 years with each child having at least 1 follow-up scan at a mean of 12.1 years of age. The mean interval between first and follow-up studies was ∼ 2 years. In this cohort, the prevalence of SCI at baseline was 21.2%.15 SCIs commonly differ in size and location compared with overt strokes. Whereas overt strokes are typically located in both cortex and deep white matter,16 SCI in the CSSCD typically occurred in the deep white matter of the frontal (81%; Figure 1E-F) or parietal (45%) lobes (Figure 2A-D), followed in frequency by the basal ganglia (Figure 1G-H) or thalamus (16%) and the temporal lobes (9%).15 Similar observations were reported for an unselected cohort of 132 children with SCA in Créteil, France undergoing brain MRI as part of routine medical care2 and in other studies.4,5,17,18 SCI in SCA are typically smaller in size compared with overt strokes. In the CSSCD participants, 83% of the children with overt strokes, but only 16% of those with SCI, had lesions that were at least 1.5 cm.15
The CSSCD results supported early observations that the presence of SCI is a risk factor for additional neurologic injury,19 with a higher risk of both clinical stroke (14-fold), and progressive silent infarction. Approximately 25% of the children with SCI, but only 2.5% of the children without SCI, had new and/or enlarging lesions on follow-up MRI scan. The incidence rates of new or more extensive SCI were 1.0 and 7.0 events per 100 patient-years, respectively, for children with or without preexisting SCI.15 SCI is most common in patients with hemoglobin SS but may also be identified in individuals with heterozygous genotypes including sickle β-thalassemia and hemoglobin SC (HbSC) disease. Approximately 3% to 38% of patients with sickle β-thalassemia,17,18,20,21 and 5% to 31% of those with HbSC disease,15,22 will have SCI. However, limited sample size allows no significant inferences about the rate of progression or potential benefit of therapy.
One of the greatest limitations in defining the epidemiology of SCI is the lack of longitudinal studies looking at incidence rate in different age groups. Existing studies do not fully address when SCI first occur in young children or whether the incidence of SCI falls in adolescents. Both the CSSCD and the French cohorts included children who were older than 6 years of age; thus, the youngest age at which SCI occurred in these 2 studies could not be determined. Three small studies have provided some estimates as to when SCI may start to occur. In a single-center study, 39 children with SCA with no history of a stroke underwent MRI between 7 and 48 months of age23 ; 4, including all 3 with a history of seizures, had SCI. In the second study, a multicenter phase 3 study (BABY HUG), MRI scans were performed in 23 infants with SCA at an average age of 13.7 months; 3 (13%) had SCI.24 In the third study, as part of routine medical care in children before their sixth birthday, 27.7% (18 of 65) of the asymptomatic children with SCA undergoing MRI had an SCI.1 In the French study, the incidence of SCI was 28.2% by age 8 years of age and 37.4% by age 14 years of age,2 suggesting absence of an incidence plateau (Figure 5), providing one of the reasons for current and future secondary and primary SCI prevention trials.
SCIs in adults with SCA
Solid estimates of the prevalence of SCI in adults with SCA are limited by both the number of studies and study participants within and across studies. In the first longitudinal study published among patients with SCI, Kugler et al enrolled 16 individuals with SCA, but no history of stroke or transient neurologic events and normal neurologic examination, to undergo MRI of the brain at a mean age of 20 years.19 The primary aim of this study was to determine the predictive value of SCI as a risk factor for clinically apparent strokes. Patients were followed for a mean of 3.7 years. Eight (50%) had SCI, of whom 4 (25% of the total) had progressive abnormalities on MRI, with 3 developing focal neurologic deficits. In the 8 patients with normal brain MRI, there was no evidence of progression on MRI or clinically. Although not a focus, this study also found that the majority of the patients had significant deficits on cognitive testing regardless of the presence of SCI.19
In another study of 50 adults, including 4 with overt stroke, Silva et al found that leukoencephalopathy was the most common abnormality (48%),25 followed by atrophy (28%; Figures 1H, 2D), encephalomalacia (6%; Figure 2F-H), and lacunes (Figure 1E-H; 4%). Vichinsky et al completed brain MRI in adults with SCA as an initial component of a randomized trial.9 Entry into the study required that the participants had a baseline hemoglobin concentration below 10 g/dL without any history of stroke or focal neurologic deficits, known brain imaging abnormalities, serious cognitive impairment, or evidence of depression. Nevertheless, in this group of highly selected adults with SCA, SCI, referred to as lacunar infarction, was present in 13% of the surveillance MRIs compared with 2% of the ethnic and age-matched controls without SCA. In contrast, white matter lesions occurred in 15% of individuals with SCA and 7% of controls, atrophy was seen in 23% of patients and 16% of controls, and a cortical infarct was detected in one individual with SCA and one control. The difference in SCI prevalence compared with the published pediatric cohorts may be related to either (1) the highly selected group of well-functioning adults with SCA or (2) a subtle, but important, variation in the definition of SCI, related either to the difference in minimum size (5 mm vs 3 mm) or to the requirement for T1 hypointensity, as well as T2 hyperintensity in the adult study. A longitudinal study that includes children, adolescents and adults with SCA is required to determine the true natural history and sequelae of SCI. Based on the high prevalence of SCI in adults with SCA, screening MRI of the brain may be considered standard care, despite the lack of evidence-based therapy, because detection of SCI may help facilitate employment or vocational options along with realistic expectations for the management of a complex chronic illness. Ultimately, better data are required to understand the clinical course and relevance of SCI in adults with SCA.
Neurocognitive outcomes associated with SCIs
Children with SCI have lower cognitive test scores compared with children with a normal MRI of the brain. Poorer global intellectual function17,22,26-28 has been reported in several studies, with function below the average range for the general population, but better than that of children with overt strokes. In a summary of global intelligence quotient in children with SCA and controls, Hogan et al graphically displayed data from multiple studies that included the Full Scale IQ (FSIQ) in controls without SCA, children with SCA with or without SCI, and children with SCA and overt stroke.29 The gradient in FSIQ demonstrated the following consistent pattern: ethnically matched control children without SCA had a mean FSIQ greater than children with SCA and without SCI, who in turn had a FSIQ greater than those with SCI and covert and overt strokes (Figure 6).29 The severity of the chronic anemia,9,17 and other factors such as the home environment, are additional factors to consider when assessing the impact of SCI on cognition in SCI cognitive outcome.
Mean Wechsler FSIQ scores obtained from children with SCD and sibling controls as reported in 8 studies. The horizontal black line represents the mean value for each category of children. FSIQ scores have a mean of 100 (SD 15). Scores above 80 are within the average range according to Wechsler classification.27 (A) Armstrong et al22 (Normal MRI, n = 105; Silent Infarct, n = 21; Clinical History of Stroke, n = 9; age range, 6-12 years). (B) Steen et al33 (Historical Control Data, n = 30; Normal cMRI, n = 12; Abnormal cMRI, n = 10; mean age, 10 years; SD, 3 years). (C) Watkins et al4 (Control, n = 15; SCD/nm, n = 15; Asymptomatic, n = 4; Symptomatic, n = 5; age range, 5-16 years). (D) Bernaudin et al17 (Siblings, n = 76; Normal MRI, n = 104; Silent Stroke, n = 17; With (Overt) Stroke, n = 11; age range, 5-15 years). (E) Brown et al31 (no cerebrovascular accident [CVA], n = 30; Silent, n = 11; CVA, n = 22; age range, 6-17 years). (F) Wang et al28 (Normal MRI, n = 122; Silent Infarct, n = 43; Stroke, n = 20; age range, 6-18 years). (G) Thompson et al64 (First Visit: Normal, n = 93; Silent Infarct, n = 29; Stroke, n = 6; age range, 5-15 years). (H) Steen et al65 (African-American Controls, n = 30; Patients, n = 30; mean age, 10 years; SD, 2.9 years).
Mean Wechsler FSIQ scores obtained from children with SCD and sibling controls as reported in 8 studies. The horizontal black line represents the mean value for each category of children. FSIQ scores have a mean of 100 (SD 15). Scores above 80 are within the average range according to Wechsler classification.27 (A) Armstrong et al22 (Normal MRI, n = 105; Silent Infarct, n = 21; Clinical History of Stroke, n = 9; age range, 6-12 years). (B) Steen et al33 (Historical Control Data, n = 30; Normal cMRI, n = 12; Abnormal cMRI, n = 10; mean age, 10 years; SD, 3 years). (C) Watkins et al4 (Control, n = 15; SCD/nm, n = 15; Asymptomatic, n = 4; Symptomatic, n = 5; age range, 5-16 years). (D) Bernaudin et al17 (Siblings, n = 76; Normal MRI, n = 104; Silent Stroke, n = 17; With (Overt) Stroke, n = 11; age range, 5-15 years). (E) Brown et al31 (no cerebrovascular accident [CVA], n = 30; Silent, n = 11; CVA, n = 22; age range, 6-17 years). (F) Wang et al28 (Normal MRI, n = 122; Silent Infarct, n = 43; Stroke, n = 20; age range, 6-18 years). (G) Thompson et al64 (First Visit: Normal, n = 93; Silent Infarct, n = 29; Stroke, n = 6; age range, 5-15 years). (H) Steen et al65 (African-American Controls, n = 30; Patients, n = 30; mean age, 10 years; SD, 2.9 years).
Specific areas of deficit have been associated with SCI, including executive functions like selective attention, card sorting, working memory, and processing speed,4,30-32 visual motor speed and coordination,4,22 vocabulary,17,22,28,33 visual memory,34 and abstract reasoning and verbal comprehension.17,35 As a consequence of these specific deficits, academic achievement in math and reading are also affected, with one study reporting that the 35% of children with SCA and SCI had twice the chance of academic difficulties as those without SCI.26
Using the definition from the CSSCD, along with a negative neurologic examination by a pediatric neurologist, the group at Washington University demonstrated that lesion location correlated with deficits attributable to the corresponding cognitive domain,36 that the volume of the SCI was associated with the magnitude of cognitive deficit,37,38 and that the presence of SCI was associated with poor academic attainment compared with controls.27 Together these data clearly demonstrate that SCI are associated with a specific cognitive profile correlating with their distribution in the frontal lobe, and are associated with cognitive deficits and academic difficulties compared with children with SCA but normal MRI as well as unaffected siblings (Figures 6 and 7).
The proportion of students with SCA with and without SCIs and sibling controls that have either failed a grade or received special services.26
The proportion of students with SCA with and without SCIs and sibling controls that have either failed a grade or received special services.26
Pathology
Large infarcts in an arterial territory, or in the border zones between anterior and middle cerebral arteries, have been described in patients dying after a documented overt stroke,39,40 in association with endothelial hyperplasia, fibroblastic reaction, hyalinization and fragmentation of the internal elastic lamina, and thrombi in large and small vessels.39 There are no autopsy studies from the neuroimaging era that have focused specifically on the pathology of subclinical brain damage, such as SCI, in SCA. Pathologies inferred to account for the typical small necrotic lesions in the border between the cortex and the subcortical white matter include acute demyelination41 and venous sinus thrombosis,39 as well as disease of the small arteries and arterioles.
Risk factors for SCIs
Only a few studies have specifically evaluated risk factors for SCI in SCA. The CSSCD compared 42 children with SCA and SCI with 188 children with SCA but without SCI. In a multivariable logistic regression, clinical risk factors associated with SCI included a history of seizures, low pain event rate, elevated white blood cell count, and the SEN βS globin gene haplotype.6 Two significant limitations should be considered, however, when interpreting these results. First, the CSSCD research team later updated their results from this same cohort and acknowledged that their adjudication process for SCI improved.15 Second, the sample size of children with SCI was relatively small, limiting the ability to detect small differences and resulting in odds ratios (ORs) with large confidence intervals (CIs). In a single-center study of the risk factors for SCI in a longitudinal prospective cohort,2 the only independent risk factor for SCI was baseline low hemoglobin level.
In the SIT Trial cross-sectional study, in which participants with epilepsy, as well as those with abnormal transcranial Doppler (TCD), were excluded, 814 children with SCI-like lesions were examined by a pediatric neurologist to confirm that the cerebral lesions were not associated with any focal neurologic deficit, a critical feature of SCI. Neuroradiology and neurology committees adjudicated the presence of SCI, which was diagnosed in 30.8% of participants. In a multivariable logistic regression analysis, the following were statistically significantly associated with an increased risk of a SCI: (1) hemoglobin in the lowest quartile, < 7.6 g/dL, compared with the highest quartile, ≥ 8.6 g/dL (OR 2.12, 95% CI 1.45-3.10, P < .001); (2) systolic blood pressure (SBP) in the highest quartile, > 113 mmHg, compared with the lowest quartile, < 104 mmHg (OR 1.73, 95% CI 1.18-2.54, P = .013); and (3) male sex compared with the reference population female sex (OR 1.42, 95% CI 1.04-1.93, P = .026).8
SCIs and cerebrovascular disease (TCD and MRA)
No established relationship exists between SCI and abnormal TCD measurements or magnetic resonance angiography (MRA) imaging. In the STOP (Stroke Prevention Trial in SCA) Trial, the prevalence of SCI at randomization to regular blood transfusion or standard treatment in those with abnormal TCD was 47 (37%) of 127 patients with SCD and abnormal TCD.42 This is similar to a recent report from France in a single-center population not selected for TCD abnormality.2 In a retrospective study of 78 children with SCA from 5 centers participating in both the CSSCD and the STOP Trial, including 17 children with SCI, Wang et al found no significant statistical relationship between the presence of SCI and abnormal TCD measurements.43 The majority of children with SCI in the context of SCD do not have evidence of cerebral vasculopathy as assessed by MRA of the circle of Willis.44 However, a larger, more systematic, analysis is needed to formally address the relationship between TCD measurements, SCI, and MRA abnormalities.
Pathophysiology
SCI is believed to be a small vessel disease, although direct evidence supporting this postulate is lacking. In fact, in 9 adults without SCA, but with carotid occlusion, small lesions were identified in the deep white matter, similar in character to SCI and likely to be related to poor cerebrovascular hemodynamic reserve.45 In SCD, focal perfusion abnormalities are common46 and are often, but not always, related to infarction (overt and silent) and cerebrovascular disease. One MR perfusion study included 48 patients, 8 and 14 with overt stroke and SCI, respectively, and 25 with perfusion abnormality. Only 4 patients with small SCI had no detectable perfusion abnormality.46 Studies exploring other reasons for reduced perfusion, including imaging of the heart and neck vessels, are required to exclude cardiac, carotid, or vertebrobasilar occlusive disease as possible mechanisms.47 The predilection for SCI to occur in the border zone areas of the brain suggests that hemodynamic factors could play a role in the pathogenesis.
Regardless of the etiology, chronic anemia is associated with SCI and lower global IQ.17,48,49 Anemia results in hyperemia, hypervolemia, and cerebral vasculature vasodilatation50 to maintain constant cerebral metabolic rate for oxygen. The intrinsic compensatory mechanisms to maintain constant global cerebral blood flow (CBF) are already close to the maximum levels, so any decrease in CBF for physiologic reasons, for example, increased carbon dioxide tension,50,51 compromised cardiac function,52 or increased metabolic demand of the brain tissue, which carries a risk of imbalance between demand and supply of oxygen to the brain. As would be anticipated, children with SCA and acute illness that results in either a decrease in oxygen delivery, such as acute chest syndrome, or an increase in metabolic demands of the brain, such as seizure or fever, would be expected to be at risk for SCI. Dowling et al demonstrated that children with SCA and normal MRA of the brain had evidence of SCI temporally associated with clinical events that included, but were not limited to, acute drops in their hemoglobin secondary to infection, decrease in oxygen delivery secondary to acute chest syndrome, or increase in the metabolic demands of the brain from fever.53 Thus, this small but important case series, along with the recent data demonstrating that baseline low hemoglobin is a risk factor for SCI,2,8 provide proof of principle that an alteration in either the delivery of oxygen (based on a low hemoglobin) or increase in cerebral metabolic demand (Figure 8), may provide sufficient cause for unsuspected SCI that will only be detected with a low threshold to perform brain MRI.54
Potentially synergistic factors leading to SCI in SCA. Modified from DeBaun et al54 with permission. Equation 1: The CMRO2 is the cerebral metabolic rate for oxygen. The brain relies on a constant delivery of oxygen to maintain CMRO2. If the delivery is inadequate, permanent tissue injury can occur, depending on the depth and duration of the ischemia. The equation above relates cerebral blood flow (CBF; the bulk delivery of blood to the brain), oxygen extraction fraction (OEF; the fraction of available oxygen blood that leaves the blood by passive diffusion as it passes through the circulation), and the arterial oxygen content (CaO2). Equation 2: CaO2, in turn, is a product of the hemoglobin content of blood and the arterial oxygen saturation. Reduced hemoglobin (Hgb; or oxygen carrying capacity of the existing Hgb) or hypoxia may reduce the arterial oxygen content. One can see the potentially synergystic effects of these relationships in reducing the oxygen delivery to the brain below critical thresholds. CaO2 can fall because of anemia and hypoxia and CBF can fall because of the large artery vasculopathy. Patients with preexisting arteriopathy may be more likely to suffer an ischemic stroke than other patients with the same degree of anemia or hypoxia.
Potentially synergistic factors leading to SCI in SCA. Modified from DeBaun et al54 with permission. Equation 1: The CMRO2 is the cerebral metabolic rate for oxygen. The brain relies on a constant delivery of oxygen to maintain CMRO2. If the delivery is inadequate, permanent tissue injury can occur, depending on the depth and duration of the ischemia. The equation above relates cerebral blood flow (CBF; the bulk delivery of blood to the brain), oxygen extraction fraction (OEF; the fraction of available oxygen blood that leaves the blood by passive diffusion as it passes through the circulation), and the arterial oxygen content (CaO2). Equation 2: CaO2, in turn, is a product of the hemoglobin content of blood and the arterial oxygen saturation. Reduced hemoglobin (Hgb; or oxygen carrying capacity of the existing Hgb) or hypoxia may reduce the arterial oxygen content. One can see the potentially synergystic effects of these relationships in reducing the oxygen delivery to the brain below critical thresholds. CaO2 can fall because of anemia and hypoxia and CBF can fall because of the large artery vasculopathy. Patients with preexisting arteriopathy may be more likely to suffer an ischemic stroke than other patients with the same degree of anemia or hypoxia.
SCI may result from additional causes beyond hemodynamic insufficiency. Some SCI may result from thromboemboli, for example, related to patent foramen ovale53 or intrapulmonary shunts, although the prevalence of SCI-associated patent foramen ovale is probably similar to that in the general pediatric population.53 The role of other mechanisms, such as posterior reversible encephalopathy syndrome,55 venous sinus thrombosis,56 and demyelination,57 in the development of SCI has not been extensively explored.
Clinical management of SCIs
Given the observation that SCI are associated with current morbidity, including poor educational attainment26 and future cerebral infarcts,15 evidence-based medical interventions are needed. However, currently no established therapy is available for primary or secondary prevention of SCI.
Children with SCA with both SCI and abnormal TCD are at higher risk of developing an overt stroke42 than those with only an abnormal TCD measurement. In the STOP Trial, comparing participants in the observation arm with both SCI and abnormal TCD measurements versus those with only an abnormal TCD measurement, the rate of a stroke was significantly greater: 52% (15 of 29) compared with 21% (9 of 42; P = .008). These results suggest that the presence of SCI in the setting of an abnormal TCD measurement is associated with increased risk of stroke. In the same study, the investigators demonstrated that blood transfusion therapy attenuated the rate of strokes in the group with both SCI and elevated TCD measurements compared with only elevated TCD measurement, 0% (0 of 18) and 5% (2 of 38) respectively. Twelve patients developed new or enlarged SCI after 36 months of follow-up, only 1 of whom was randomized to transfusion. These results provided preliminary evidence that regular blood transfusion therapy may be effective in preventing neurologic injury and served as strong preliminary data for the SIT Trial.7 In a follow-up study, the results were similar. At the end of STOP2, when children with elevated TCD measurements were randomized to continue or to stop transfusion, 8% of participants who continued transfusion developed new brain MRI lesions compared with 28% who stopped. The total number of lesions decreased from 25 to 24 in the continued-transfusion group while increasing from 27 to 45 in those who stopped.58 Taken together, data from both STOP and STOP2 indicated that children with SCA and both SCI and elevated TCD measurements are at extremely high risk to have progressive SCI. However, we do not know whether children with SCA, SCI, and elevated TCD measurements below the transfusion threshold are at increased risk of SCI and if so, whether the magnitude of the risk outweighs the burden and benefit of regular blood transfusion therapy.
Based on the high prevalence of SCI, the presence of cognitive and educational morbidity, coupled with the progressive nature of SCI, King et al performed a single-arm feasibility study demonstrating that parents were willing to accept blood transfusion therapy as an intervention.59 These results also provided preliminary evidence for the short-term efficacy of blood transfusion therapy to prevent progression of neurologic injury in patients with SCI. Subsequently, the SIT Trial (NCT00072761) was funded.7 The primary hypothesis of the SIT Trial is that children randomly allocated to blood transfusion will have an 86% relative risk reduction of an overt stroke or progressive SCI compared with children randomly allocated to observation.7 A total of 1211 children with SCA between the ages of 5 and 15 years were screened for SCI, of whom 196 were randomly allocated to either arm. The study is fully enrolled; although, a 3-year follow-up is required, thus results from this trial are not expected until after June 2013.
No other systematic strategy for the treatment of SCI has been established. Hydroxyurea and HSCT are potential options for primary and secondary prevention of SCI. There are preliminary data for both therapies in single-arm studies, suggesting efficacy as a secondary preventive strategy for SCI. Two groups have documented that no new SCI have occurred after successfully engrafted HSCT57,60 although one HSCT study documented that new SCI may occur in the immediate posttransplantation period.61 No clinical trial has been undertaken to determine the relative benefits and burden of HSCT as secondary prevention of SCI.
Recently, in a prospective multicenter, single-arm study, Hulbert et al demonstrated that children with overt strokes receiving blood transfusion therapy may still develop new SCI.62 Forty children with overt strokes were followed for ∼ 5 years with surveillance MRI and MRA performed every 1-2 years. The mean pretransfusion hemoglobin S concentration was < 30% throughout the treatment period. Approximately two-thirds of the patients had MRA evidence of cerebral vasculopathy at baseline. Despite the rigorous transfusion regimen, 18% (7 of 40) and 28% (11 of 40) had new overt or SCI, respectively. These data strongly challenge the paradigm that blood transfusion therapy is an optimal therapy for secondary prevention of stroke or SCIs when there is preexisting vasculopathy. Furthermore, for children with overt strokes who are receiving blood transfusion therapy, surveillance MRI of the brain every 1-2 years should be done to assess whether there is progressive neurologic disease because as many as 25% of this population may develop new SCI,62 and alternative therapies, for example, revascularization for moyamoya, might be considered.
Screening for SCIs
Despite the lack of evidence for an established medical treatment for SCI, there is sufficient support that identifying SCI is of clinical benefit to the child with SCA. Screening for SCI is currently performed in selected hematology centers where multidisciplinary teams are evaluating cognitive services and medical interventions, for example, hydroxyurea or HSCT for children with SCA and infarcts of the brain.1,2,63 Based on the high prevalence of SCI, at least 27% before 6 years of age, coupled with the well-established cognitive deficits, performing screening MRI examination in students at least once is a reasonable strategy. Early diagnosis of a SCI in a child with cognitive or behavioral problems may enable teachers to alter strategies and may secure additional education resources to help the student gain the skills necessary for successful academic attainment. Services include, but may not be limited to, occupational therapy, parent counseling, physical therapy, social work services, speech/language pathology, assistive technology, recreation, and counseling services. In the United States, special services are provided in a least restrictive educational setting and are designed specific to the needs of the students through Individual Education Programs (IEPs). Eligibility for special education services is identified by the school through a full evaluation of the child in all areas of suspected disability. If the student is found eligible for services, the school is required to convene the IEP team annually and develop an appropriate plan for the child. The presence of SCI in children with SCA, coupled with significant deficits on cognitive test scores, greatly increases the likelihood of obtaining an IEP in the United States or a statement of educational needs in Europe. Similar strategies, namely assessment of resources for adults with SCI, have not been studied.
Summary
Among children with SCA, SCI are prevalent and associated with significant cognitive and academic morbidity. SCI is a distinct clinical entity compared with overt stroke (see Table 1). The natural history and risk factors for SCI are incompletely understood. SCIs occur in infants as young as 1 year of age and continue throughout childhood with the highest incidence rate in children < 6 years of age. The frequency and natural history of new SCI occurring among adults is not well defined. Among individuals with preexisting SCI, there is a significant risk for new SCI or overt strokes compared with individuals without SCI. The only method available to reliably identify SCI is brain MRI. Children with SCD who perform poorly in the classroom or have an acute decline in academic performance should be considered for evaluation for SCI using MRI, a neurologic examination, and cognitive testing. Adults with SCA have at least a one-third chance of having a SCI. Given the high neurologic disease burden, screening and detection of SCI has become standard care where supportive services are readily available. For children with SCA and SCI, an ongoing multicenter international trial will determine whether blood transfusion therapy is an efficacious treatment in preventing further neurologic injury compared with observation.7 Until the results of this trial are known, the use of transfusions should not be considered a standard of care and only considered on a case-by-case basis. Likewise, there is no high-level evidence to support the use of hydroxyurea or HSCT for SCI. Future work should focus on the risk factors and potential primary prevention of SCI, particularly in the highest risk group, young children between infancy and 6 years of age.
Clinical characteristics of overt strokes and silent cerebral infarcts in sickle cell anemia
| . | Overt strokes . | Silent cerebral infarcts . |
|---|---|---|
| Frequency prior to 6th birthday | 2%-5% | 27% |
| Mean global IQ | 71 | 83 |
| Primary prevention | Transcranial Doppler followed by blood transfusion therapy | None established; ongoing limited institution studies of hydroxyurea and HLA-matched sibling transplantation |
| Secondary prevention | Blood transfusion therapy HLA-matched sibling transplant revascularization for moyamoya | None established; await the results of the Silent Cerebral Infarct Transfusion Trial |
| . | Overt strokes . | Silent cerebral infarcts . |
|---|---|---|
| Frequency prior to 6th birthday | 2%-5% | 27% |
| Mean global IQ | 71 | 83 |
| Primary prevention | Transcranial Doppler followed by blood transfusion therapy | None established; ongoing limited institution studies of hydroxyurea and HLA-matched sibling transplantation |
| Secondary prevention | Blood transfusion therapy HLA-matched sibling transplant revascularization for moyamoya | None established; await the results of the Silent Cerebral Infarct Transfusion Trial |
Acknowledgments
The authors thank Diana Truran-Sacrey, MD, for initial comments before preparing the manuscript; Mark Roedigher for preparing Figure 5; and Cindy Terrill for manuscript preparation.
This work was supported by funding from the National Institute of Neurological Disorders and Stroke (U01 NS042804; M.R.D.); Burroughs Wellcome Translational Research Award no. 1006671 (M.R.D.); Action Medical Research (F.J.K.); and The Wellcome Trust (0353521/A/92&94/Z and 056325/Z/98; F.J.K.).
National Institutes of Health
Wellcome Trust
Authorship
Contribution: M.R.D., F.D.A., R.C.M., R.E.W., E.V., and F.J.K. analyzed the data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael R. DeBaun, Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN 37232; e-mail: m.debaun@vanderbilt.edu.





![Figure 6. Mean Wechsler FSIQ scores obtained from children with SCD and sibling controls as reported in 8 studies. The horizontal black line represents the mean value for each category of children. FSIQ scores have a mean of 100 (SD 15). Scores above 80 are within the average range according to Wechsler classification.27 (A) Armstrong et al22 (Normal MRI, n = 105; Silent Infarct, n = 21; Clinical History of Stroke, n = 9; age range, 6-12 years). (B) Steen et al33 (Historical Control Data, n = 30; Normal cMRI, n = 12; Abnormal cMRI, n = 10; mean age, 10 years; SD, 3 years). (C) Watkins et al4 (Control, n = 15; SCD/nm, n = 15; Asymptomatic, n = 4; Symptomatic, n = 5; age range, 5-16 years). (D) Bernaudin et al17 (Siblings, n = 76; Normal MRI, n = 104; Silent Stroke, n = 17; With (Overt) Stroke, n = 11; age range, 5-15 years). (E) Brown et al31 (no cerebrovascular accident [CVA], n = 30; Silent, n = 11; CVA, n = 22; age range, 6-17 years). (F) Wang et al28 (Normal MRI, n = 122; Silent Infarct, n = 43; Stroke, n = 20; age range, 6-18 years). (G) Thompson et al64 (First Visit: Normal, n = 93; Silent Infarct, n = 29; Stroke, n = 6; age range, 5-15 years). (H) Steen et al65 (African-American Controls, n = 30; Patients, n = 30; mean age, 10 years; SD, 2.9 years).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/20/10.1182_blood-2011-02-272682/4/m_zh89991288790006.jpeg?Expires=1765895205&Signature=KMM5fn~XpniApjGKZLjp4VcJwJcLvR5WXIx0IJ7LrSoHPk-LFZscMd1Ls5U27W6y2gXqFt~1IveY2vWd6AG0mvAwS-~AYuIUm2ABcXbHAZMapaogWSMPV8U001sjk4W2rZUi3k9bcY86YEE48SOSFTETrowvPfRNd1NO2kzQbmFzvG8isQlS9I1YHE0oXQCFe57GPpabj6B4GFjCG7u5XyQ6AuiAsajOnoBVTk0wxfDo77a~NI6kO084ktYqQ8VUJHGUgpZN6FyIROHO7Rj6l9KvC-5exrENKQjpErGTUM5C~u-x3PGCDJnAxrERjdJVEA3ywHiKb~e0vMqzN8A6qw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

