Infection of erythrocytes with the human malaria parasite, Plasmodium falciparum, results in dramatic changes to the host cell structure and morphology. The predicted functional localization of the STEVOR proteins at the erythrocyte surface suggests that they may be involved in parasite-induced modifications of the erythrocyte membrane during parasite development. To address the biologic function of STEVOR proteins, we subjected a panel of stevor transgenic parasites and wild-type clonal lines exhibiting different expression levels for stevor genes to functional assays exploring parasite-induced modifications of the erythrocyte membrane. Using this approach, we show that stevor expression impacts deformability of the erythrocyte membrane. This process may facilitate parasite sequestration in deep tissue vasculature.
Introduction
Erythrocytes infected with the human malaria parasite, Plasmodium falciparum, undergo dramatic changes in structure and morphology, in part because of the export of a broad repertoire of parasite proteins as well as influences of the shape and volume of the developing parasite itself. Alterations of the erythrocyte membrane include the appearance of cytoadherent knobs, the acquisition of novel adhesive and serologic properties, an increase in membrane rigidity, and the activation of solute permeability pathways, termed the new permeability pathways, for nutrient uptake and waste removal. The increased rigidity and adhesive properties of infected erythrocytes are major factors in the survival and virulence of the parasite.1 Uninfected erythrocytes are highly deformable because of their high surface area-to-volume ratio and the elasticity of the erythrocyte membrane and cytoskeleton.2 In contrast, infection with P falciparum results in a loss of deformability, and this perhaps increases the pathogenesis of the parasite by facilitating sequestration of infected erythrocytes and blockage of microcapillaries.3
The knob-associated parasite protein KAHRP has been shown to associate with the erythrocyte cytoskeletal proteins spectrin, actin, and ankyrin, and these interactions are correlated with increased membrane rigidity.4 In cultured parasite lines, truncations of nonessential telomeric regions are well documented, and one such shortening of P falciparum chromosome 2 leads to loss of KAHRP and, as a consequence, a knobless phenotype.5 KAHRP(−) parasites propagate in culture at normal rates, supporting a role for rigidity and adhesion solely during in vivo infections. Targeted gene deletion of KAHRP, as well as knockout of another parasite gene, PfEMP3, results in a significant decrease in erythrocyte rigidity; however, the observed deformability remains less than that of uninfected erythrocytes, indicating that other factors also contribute to parasite-infected erythrocyte rigidity.4,6 The erythrocyte-exported RESA protein has been shown to contribute to the increased rigidity of ring-stage infected erythrocytes, although not of late-stage infected erythrocytes, when RESA is not expressed.7 The complexity of parasite-induced rigidity is additionally inferred by the observation that different parasite isolates of both knobby and knobless phenotype show variations in the extent of rigidity of the infected erythrocyte.8 Thus, it is probable that a repertoire of parasite proteins trafficked to the erythrocyte membrane play a structural role in increased rigidity and reduced cell deformability.
Annotation of the P falciparum proteins harboring predicted PEXEL/HT protein motifs mediating export to the erythrocyte reveals a catalog that is dominated by highly amplified gene families, such as the PHIST-domain containing proteins and members of the 2TM superfamily, namely, RIFIN, STEVOR, and Pfmc-2TM.9,–11 Members of the 2TM superfamily traffic via the Maurer's clefts to the erythrocyte membrane.12,13 The 2TM superfamily proteins possess a predicted architecture of namesake 2 transmembrane (2TM) domains flanking a region that is hypervariable between paralogs and across parasite isolates. The diversity within this predicted loop region suggests that it is exposed on the erythrocyte surface and that immune pressure drove gene amplification and selection for antigenic diversity, thereby underpinning the capacity for antigenic variation of members of the 2TM family.13 In support of this hypothesis, it has been shown by quantitative PCR that only a subset of the Pfmc-2TM and stevor genes are expressed in each parasite and that expression is clonally variant, with members of these families undergoing switching.5 Evidence of clonal and antigenic variation has also been demonstrated at the protein level using antibodies that recognize specific STEVOR proteins.14
The function of the 2TM proteins at the erythrocyte membrane remains unclear, and studies have predominantly relied on gene expression and immunolocalization methods. In the case of the stevor family, gene expression peaks at 22 to 28 hours post invasion (hpi), and protein expression has been detected in the trophozoite to schizont stages as well as in the gametocyte and sporozoite stages.5,13,–15 It has also been shown that STEVOR proteins are expressed in the apical complexes of merozoites and thus may play a role during erythrocyte invasion.16,–18 Functional assays to study the 2TM proteins are limited because of the large nature of these families, which precludes traditional knockdown strategies. In the present study, we sought to determine the contribution of STEVOR proteins to erythrocyte membrane alterations of mechanical properties using clonal lines that express specific stevor genes as well as parasite lines that overexpress or down-regulate members of the stevor gene family. We found that increased stevor expression levels result in decreased deformability of the infected erythrocyte.
Methods
Parasite culture and synchronization
The P falciparum isolate NF54 was used to generate clonal and transgenic lines (see supplemental Table 1 for description of parasite lines, available on the Blood Web site; see the Supplemental Materials link at the top of the article) and cultivated in vitro as described19 using RPMI 1640 medium supplemented with 10% heat-inactivated human serum and human erythrocytes at a 5% hematocrit. Cultures were synchronized by the isolation of schizont-stage parasites using a Percoll-sorbitol gradient, or by magnetic isolation using a MACS depletion column (Miltenyi Biotec) in conjunction with a magnetic separator, and placed back into culture. After invasion of merozoites, alanine synchronization was used for the selection of ring-stage parasites to obtain a tighter window of synchronization.20 The length of the complete asexual life cycle for each parasite line was determined by monitoring the time from one invasion to the next invasion event. For RNA isolation, parasite cultures were harvested at specific time points after erythrocyte invasion, pelleted by centrifugation, and lysed with 0.1% saponin in PBS. For stevor and Pfmc-2TM gene expression, parasites were harvested during the early to mid-trophozoite stage, at 22 to 28 hpi. Thin smears of blood were made at each time point and stained with Giemsa reagent for microscopic assessment of parasite development.
Microsphere matrices and sorting
As recently described,21 calibrated metal microspheres (96.50% tin, 3.00% silver, and 0.50% copper; Industrie des Poudres Sphériques) with 2 different size distributions (5- to 15-μm-diameter and 15- to 25-μm-diameter; each from a single batch) composed a matrix used to assay infected erythrocyte deformability under flow. Briefly, 2 g of dry microspheres of each size range was mixed and resuspended in complete medium (RPMI-10% human serum). From this microsphere suspension, 600 μL was pipetted into an inverted 1000-μL anti–aerosol filter pipet tip (Neptune; BarrierTips) and allowed to settle into a 5- to 6-mm-thick microsphere layer above the filter. Suspensions of infected erythrocytes were introduced upstream of the microsphere layer; specifically, 600 μL of a 2% hematocrit infected erythrocyte sample from defined time points of synchronized cultures. The suspensions contained less than 10% of potentially “retainable” infected erythrocytes to avoid saturation of the microsphere matrix. Infected erythrocytes were perfused through the microsphere matrix at a flow rate of 60 mL/h using an electric pump (Syramed μsp6000, Arcomed′ Ag), followed by a wash of 6 mL complete medium. The upstream and downstream samples were collected and smeared onto glass slides for staining with Giemsa reagent, and parasitemia was assayed to determine parasite retention versus flow-through. The retention rates over the course of the parasite life cycle were fitted using logistic regression (one model for each parasite line), with R and the drc package; then an ANOVA test was used to assess the statistical significance differences between the models.
Indirect immunofluorescence microscopy
Infected erythrocytes were stained with anti–c-myc FITC-conjugated mouse monoclonal antibody (Sigma-Aldrich). Details are available in supplemental Methods. Samples were analyzed using an Olympus BX-51 fluorescence microscope at 100× magnification with an Optronics digital imaging system.
RNA isolation and transcript expression analysis by real-time RT-PCR
RNA was isolated from infected erythrocytes using Trizol (Invitrogen) according to the manufacturer's instructions. Real-time PCR was performed using an ABI Prism 7900HT sequence detector (Applied Biosystems). Relative quantification of cDNA was performed using the standard curve method (User Bulletin 2, ABI; http://www.appliedbiosystems.com). Gene-specific stevor primers used to profile the expression of all stevor genes in the parasite lines were published by Lavazec et al.5 Details are available in supplemental Methods.
Ektacytometry measurement of erythrocyte population elongation index
Uninfected and infected erythrocyte elongation index was measured over a range of shear stresses (0.3-30 Pa) by ektacytometry using a laser-assisted optical rotational cell analyzer (LORCA, Mechatronics) as described.22 Uninfected erythrocytes were mock cultured in separate flasks and treated with the same conditions as the infected erythrocyte cultures. Infected erythrocytes were purified by magnetic isolation using a MACS depletion column (Miltenyi Biotec) in conjunction with a magnetic separator and placed in media for 2 hours before measurements to allow the infected erythrocytes to recover.23 The extent of erythrocyte deformability, or elongation index, was defined as the ratio between the difference of the 2 axes of the ellipsoid diffraction pattern and the sum of these 2 axes.
Scanning electron microscopy
Late trophozoite and schizont stage-infected erythrocytes were purified by magnetic isolation, and the cell pellets were resuspended in 2.5% gluteraldehyde (EM grade) in sodium cacodylate 0.1M, pH 7.2, for 1 hour at 4°C. Cells were washed 3 times in sodium cacodylate, transferred to polylysine-coated coverslips, and incubated 1 hour in 1% osmium tetroxide. After 3 washes in H2O, samples were dehydrated (25%, 50%, 75%, 95%, 2 × 100% ethanol, 5 minutes each), incubated for 10 minutes in acetone, subjected to critical point drying, and coated with palladium gold in a gun ionic evaporator. Samples were examined and photographed with a JEOL 6700 F electron microscope operating at 2 kV.
Results
Endogenous levels of stevor expression affect erythrocyte deformability
To determine possible influences on rigidity of parasite-exported 2TM proteins present at the erythrocyte membrane, we used parasite clones of NF54 that were previously characterized for stevor expression.5 In particular, we picked lines that either expressed one or several stevor genes or did not express any stevor genes (supplemental Table 1, parasite lines description). Peak stevor expression occurs at 22 to 26 hpi, with protein expression occurring during the mature parasite stages. To measure changes in erythrocyte rigidity, we used a recently developed in vitro system for measuring erythrocyte deformability of parasitized cells flowing through a defined matrix of microspheres.21 In this microsphere system, increased retention rates correspond to decreased erythrocyte deformability and vice versa. Cultures were tightly synchronized to obtain a narrow window of invasion time, and were assayed at 34 hpi for erythrocyte deformability. Erythrocytes infected with the B3 and H4 clones, which express one or 2 stevor genes, displayed increased retention rates and rigidity levels (Figure 1A). In contrast, erythrocytes infected with B3B1 parasites, a subclone of B3, which does not express stevor genes, and the A12 and E10 clones, which exhibit stevor expression well below housekeeping gene expression levels, displayed significantly lower retention rates, indicating greater erythrocyte deformability at the assayed time point (Figure 1A). The overall difference in retention rates between stevor-expressing and nonexpressing clones was highly significant, indicating a strong correlation between stevor expression and increased erythrocyte rigidity (P = .0000004; Figure 1B). To more closely examine changes in retention rates over the course of the parasite life cycle, synchronized B3 and B3B1 cultures were assayed every 6 hours from 10 to 40 hpi (Figure 1C). B3B1-infected erythrocytes displayed a marked decrease in retention rates from the trophozoite stage onwards, consistent with the timeline of STEVOR protein expression.
Endogenous levels of stevor expression affect erythrocyte deformability. (A) Retention rates in microsphere matrices at 34 hpi for erythrocytes infected with the NF54 clonal lines B3, H4, B3B1, E10, and A12. Gray represents clones exhibiting significant expression of a least one stevor gene; and red, stevor nonexpressing clones. Measurements were performed on 2% hematocrit cultures containing 2% to 10% tightly synchronized parasites. (B) Average retention rate in microsphere matrices at 34 hpi for erythrocytes infected with clones that do not express stevor genes (red) is significantly different from that of clones expressing at least one stevor gene (gray), as measured by a Wilcoxon rank-sum test. (C) Kinetics of retention in microsphere matrices for B3 (gray) and B3B1 (red) parasite lines during parasite life cycle. Measurements were performed at 10, 16, 22, 28, 34, and 40 hpi on 2% hematocrit cultures containing 2% to 10% tightly synchronized parasites.
Endogenous levels of stevor expression affect erythrocyte deformability. (A) Retention rates in microsphere matrices at 34 hpi for erythrocytes infected with the NF54 clonal lines B3, H4, B3B1, E10, and A12. Gray represents clones exhibiting significant expression of a least one stevor gene; and red, stevor nonexpressing clones. Measurements were performed on 2% hematocrit cultures containing 2% to 10% tightly synchronized parasites. (B) Average retention rate in microsphere matrices at 34 hpi for erythrocytes infected with clones that do not express stevor genes (red) is significantly different from that of clones expressing at least one stevor gene (gray), as measured by a Wilcoxon rank-sum test. (C) Kinetics of retention in microsphere matrices for B3 (gray) and B3B1 (red) parasite lines during parasite life cycle. Measurements were performed at 10, 16, 22, 28, 34, and 40 hpi on 2% hematocrit cultures containing 2% to 10% tightly synchronized parasites.
To verify stevor expression levels, quantitative RT-PCR analysis was performed using RNA isolated at 22 to 26 hpi from the cultures used in the rigidity experiments (supplemental Figure 1). This confirmed the predominant expression of at least one stevor in B3 and H4 lines but lack of significant stevor expression in B3B1, A12, and E10. The presence of the KAHRP locus was additionally determined and, as previously shown, the B3 and B3B1 lines harbor a deletion of chromosome 2 and lack KAHRP and PfEMP3.5 Although both KAHRP and PfEMP3 are involved in infected erythrocyte rigidity, they cannot account for the differences seen between the B3 and B3B1 clones that are genetically identical yet display significant differences in retention rates. The A12 clone possesses a deletion in the left arm of chromosome 7, which encompasses members of the multigene families var, rifin, stevor, and phist, for which deletions are often seen in cultured clonal lines and are tolerated because of the presence of other family members.5 The H4 and E10 lines do not have any known deletions of the chromosomal ends compared with the genome of the 3D7 isolate.
STEVOR proteins are involved in P falciparum–infected erythrocyte rigidity
If STEVOR proteins reduce erythrocyte deformability, then overexpression of a STEVOR may further enhance this phenotype above levels seen with endogenous STEVOR expression in cultured lines. To test this hypothesis, we used a transgenic parasite line, termed SFM+ (STEVOR-Flag-c-myc),13 which was generated in our laboratory to overexpress the PFF1550w stevor gene. We additionally monitored the effect of down-regulated stevor expression on erythrocyte deformability, using the SB3 line, which was derived from the B3 clonal line and displays an epigenetic knockdown of all endogenous stevor genes because of the episomal overexpression of the blasticidin resistance gene driven via a stevor promoter.24 In this line, culturing in the presence of a high concentration of blasticidin (10 μg/mL) up-regulates the activity of the episomal stevor promoter upstream of the blasticidin resistance gene. This in turn results in the down-regulation of all endogenous stevor promoters, presumably because of the recruitment and titration of factors required for stevor-specific transcription. The CBM-BSD line, which expresses an episomal copy of the blasticidin resistance gene under the control of the hrp3 promoter, served as a control for the possible effects of blasticidin drug at the concentration used in these experiments.
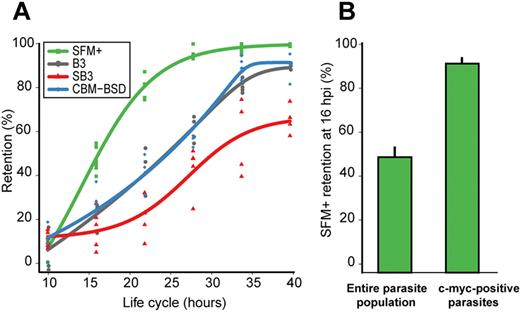
Analysis of retention rates of synchronized cultures over the course of the parasite life cycle showed a remarkable increase in retention of the SFM+ overexpressing line starting at the ring stage when the hrp3 promoter is active (Figure 2A). Retention rates for SFM+ parasite-infected erythrocytes were significantly higher (ANOVA: df = 1, F = 21.7658, P = .0001) than for the B3 line exhibiting endogenous levels of stevor expression, indicating enhanced erythrocyte rigidity. Transcript levels of the SFM+ line at 22 hpi were assayed via quantitative PCR and verified an increase in expression of PFF1550w above housekeeping gene levels (10- to 15-fold increase; supplemental Figure 4). As a control for expression of a gene from the hrp3 promoter of the parent pHL plasmid, parallel experiments for retention of erythrocytes infected with the pHL-Pfmc–2TMFM (Pfmc–2TM-Flag–c-myc) transgenic parasite line were conducted at all time points.13 Retention rates for pHL-Pfmc-2TMFM–infected erythrocytes were not increased compared with that of the B3 line, indicating that the increased retention rates seen in the SFM+ line are not related to the overexpression of any erythrocyte membrane trafficked parasite protein from this plasmid (supplemental Figure 2) but rather to a process specifically linked to stevor overexpression.
STEVOR proteins are involved in P falciparum–infected erythrocyte rigidity. (A) Kinetics of retention in microsphere matrices for B3 (gray), SFM+ (green), SB3 (red), and CBM-BSD (blue) parasite cultures during the parasite life cycle. Measurements were performed at 10, 16, 22, 28, 34, and 40 hpi on 2% hematocrit cultures containing 2% to 10% tightly synchronized parasites. (B) Retention rate in microsphere matrices at 16 hpi of the entire SFM+ parasite-infected erythrocyte population compared with that of the c-myc–positive SFM+ parasites. The proportion of c-myc–positive parasites before and after microsphere filtration was determined by immunofluorescence using anti–c-myc mAb and Hoechst 33342 for nuclei staining.
STEVOR proteins are involved in P falciparum–infected erythrocyte rigidity. (A) Kinetics of retention in microsphere matrices for B3 (gray), SFM+ (green), SB3 (red), and CBM-BSD (blue) parasite cultures during the parasite life cycle. Measurements were performed at 10, 16, 22, 28, 34, and 40 hpi on 2% hematocrit cultures containing 2% to 10% tightly synchronized parasites. (B) Retention rate in microsphere matrices at 16 hpi of the entire SFM+ parasite-infected erythrocyte population compared with that of the c-myc–positive SFM+ parasites. The proportion of c-myc–positive parasites before and after microsphere filtration was determined by immunofluorescence using anti–c-myc mAb and Hoechst 33342 for nuclei staining.
As a further indication of this specific link, erythrocytes infected with the SB3 line, which displayed no detectable stevor expression under blasticidin pressure, showed markedly reduced retention rates compared with B3 parasite-infected erythrocytes (ANOVA: df = 2, F = 19.405, P = .00001), indicating reduced rigidity. The absence of stevor expression was verified in this line by quantitative PCR using gene-specific primers for the full repertoire of stevor genes (supplemental Figure 3). Erythrocytes infected with the blasticidin control line, CBM-BSD, derived from the B3 clonal line, displayed similar rates of retention as B3, indicating that the phenotype of the SB3 line was unrelated to blasticidin drug treatment. Apart from the SB3 line, the lines assayed in these experiments reached similar levels of retention at 40 hpi, with the SFM+ line showing the highest retention rate.
Ektacytometry analysis of deformability in parasite lines
In a parallel approach, we tested the deformability of erythrocytes infected with our parasite lines using LORCA ektacytometry. In these experiments, the elongation index of infected erythrocytes is measured in response to exposure to increasing shear stress, with higher elongation indices corresponding to increased erythrocyte deformability. Uninfected erythrocytes are generally more deformable and less rigid, and thus display higher elongation indices than infected erythrocytes.22 To measure deformability changes induced by endogenous stevor expression, infected erythrocytes were purified via the MACS column system from 32 hpi cultures of B3 and SB3 lines, for comparison with uninfected erythrocytes. Purified infected erythrocytes were placed back in media at 50% parasitemia for 2 hours, and at 34 hpi these samples were subjected to LORCA measurements. As expected, uninfected erythrocytes were highly deformable and displayed the highest elongation indices. SB3-infected erythrocytes consistently showed higher elongation indices over all shear stresses measured compared with B3-infected erythrocytes because of increased erythrocyte deformability (Figure 3A). These results validate the conclusions drawn from the microsphere experiments that the SB3 line, which does not express stevor, is less rigid than the stevor-expressing B3 line.
Ektacytometry analysis of erythrocyte elongation index in parasite lines. LORCA measurements of uninfected erythrocytes (black) compared with erythrocytes infected with wild-type B3 (gray) or SB3 (red) parasite lines at 34 hpi (A), and to erythrocytes infected with B3 or SFM+ (green) parasite lines at 28 hpi (B). Erythrocyte deformability is expressed as elongation index and is determined at increasing shear stress, measured in Pascal (Pa). Parasites were synchronized by MACS and concentrated to 50% parasitemia to increase sensitivity of the measurement. Error bars represent SD.
Ektacytometry analysis of erythrocyte elongation index in parasite lines. LORCA measurements of uninfected erythrocytes (black) compared with erythrocytes infected with wild-type B3 (gray) or SB3 (red) parasite lines at 34 hpi (A), and to erythrocytes infected with B3 or SFM+ (green) parasite lines at 28 hpi (B). Erythrocyte deformability is expressed as elongation index and is determined at increasing shear stress, measured in Pascal (Pa). Parasites were synchronized by MACS and concentrated to 50% parasitemia to increase sensitivity of the measurement. Error bars represent SD.
Because stevor expression is much higher in the SFM+ line than the B3 line, it might be expected that erythrocytes infected with the SFM+ line would show a greater reduction in deformability and correspondingly lower elongation indices. Because the greatest difference in erythrocyte rigidity between SFM+ and the other lines via the microsphere matrix methodology was seen at 28 hpi, this time point was selected for analysis via LORCA. As expected, there was a marked difference between the B3 and SFM+ lines; specifically, erythrocytes harboring the SFM+ line were more rigid and had consistently lower elongation indices over all shear stresses tested (Figure 3B). Because LORCA measures the elongation index for a population of erythrocytes, we cannot determine directly whether the membrane itself is more rigid as could be determined by single-cell techniques; however, we can conclude that both the LORCA and the microsphere methodologies are consistent with a correlation between stevor expression and the decreased deformability of infected erythrocytes.
Loss of retention after episomal shedding of SFM+ line
Because the SFM+ line overexpresses stevor by virtue of an episomal cassette, some parasites fail to retain the episome during cell division, resulting in approximately 70% positivity of c-myc epitope-tagged STEVOR expression by immunofluorescence microscopy of Hoechst-positive infected erythrocytes. To determine whether a higher proportion of SFM+ parasites were retained in the microsphere matrix, we performed immunofluorescence assays on the upstream and downstream samples of a 16-hpi culture filtered through the microspheres. We found virtually no c-myc–positive parasites in the downstream samples (Figure 2B). This result also validates the role of STEVOR expression in decreased erythrocyte deformability.
Loss of episomally derived STEVOR expression within the SFM+ line is expected to result in a reversion to the levels of retention associated with endogenous stevor expression. To promote shedding of the episomal cassette, the SFM+ parasite line was cultured for 21 generations in the absence of pyrimethamine, leading to a new line called SFM−. Immunofluorescence assays on SFM− parasites using antibodies against the c-myc epitope-tagged STEVOR protein were negative, indicating that the episome harboring the stevor expression cassette had been lost (Figure 4A). Retention rates were then assayed for erythrocytes infected with this SFM− line and compared with the SFM+ and B3 cultures. The genetic background of the SFM− line is identical to the SFM+ line, so any changes in retention are probably solely the result of the presence or absence of high levels of expression of the PFF1550w STEVOR. Indeed, the B3, SFM+, and SFM− lines were checked by scanning electron micrographs to confirm the absence of KAHRP-mediated knobs in B3, and their presence in both the SFM+ and SFM− lines (Figure 4B). Retention rates were measured every 6 hours over the parasite life cycle, starting from 10 to 40 hpi, and erythrocytes infected with the SFM− line showed a significant decrease in retention rates (ANOVA: df = 3, F = 3.595, P = .032) compared with the SFM+ overexpressing line (Figure 4C). Retention rates of the SFM− line-infected erythrocytes were similar to B3, though slightly higher. This small difference in retention between the SFM− and B3-infected erythrocytes could be accounted for by the presence of KAHRP in the SFM− line (and its absence in the B3 line), a parasite-encoded protein that was previously shown to increase the rigidity of parasite-infected erythrocytes. Therefore, the increased rigidity of the SFM+ overexpressing line compared with the SFM− line is probably the result of the presence of high STEVOR expression, as the two lines are otherwise genetically identical.
Loss of retention after shedding of episome in the SFM+ line. (A) Immunofluorescence analysis of the SFM+ parasite line cultured during 21 generations in the presence (SFM+, top panel) or absence (SFM−, bottom panel) of 40nM pyrimethamine. Infected erythrocytes were stained with anti–c-myc mAb followed by anti–rat Alexa-488–conjugated IgG, and parasite nuclei were counterstained with Hoechst 33342. Pictures were taken under identical exposure conditions. Absence of c-myc expression in SFM− line indicates a loss of episomal expression of the epitope-tagged STEVOR protein. (B) Scanning electron micrograph of B3, SFM+, and SFM− parasite-infected erythrocytes. Right and middle panels: SFM− and SFM+ infected erythrocytes with normal knobs compared with erythrocyte infected with the KAHRP-deficient B3 parasite line (left panel) in which knobs are absent. The bars represent 2 μm. (C) Kinetics of retention in microsphere matrices for erythrocytes infected with B3 (gray line), SFM+ (green continuous line), and SFM− (green dotted line) during the parasite life cycle. Measurements were performed at 10, 16, 22, 28, 34, and 40 hpi on 2% hematocrit cultures containing 2% to 10% tightly synchronized parasites.
Loss of retention after shedding of episome in the SFM+ line. (A) Immunofluorescence analysis of the SFM+ parasite line cultured during 21 generations in the presence (SFM+, top panel) or absence (SFM−, bottom panel) of 40nM pyrimethamine. Infected erythrocytes were stained with anti–c-myc mAb followed by anti–rat Alexa-488–conjugated IgG, and parasite nuclei were counterstained with Hoechst 33342. Pictures were taken under identical exposure conditions. Absence of c-myc expression in SFM− line indicates a loss of episomal expression of the epitope-tagged STEVOR protein. (B) Scanning electron micrograph of B3, SFM+, and SFM− parasite-infected erythrocytes. Right and middle panels: SFM− and SFM+ infected erythrocytes with normal knobs compared with erythrocyte infected with the KAHRP-deficient B3 parasite line (left panel) in which knobs are absent. The bars represent 2 μm. (C) Kinetics of retention in microsphere matrices for erythrocytes infected with B3 (gray line), SFM+ (green continuous line), and SFM− (green dotted line) during the parasite life cycle. Measurements were performed at 10, 16, 22, 28, 34, and 40 hpi on 2% hematocrit cultures containing 2% to 10% tightly synchronized parasites.
Switching of stevor expression in B3B1 clone results in changes in erythrocyte rigidity
Over the course of in vitro culturing, switching of stevor expression can occur that is visualized after cloning of parasites and determining the expression profile for the repertoire of stevor genes.5 In this study, it was attempted to minimize switching events by minimally expanding cultures after thawing. Such potential switching events were monitored in our experiments by routine profiling of stevor expression from isolated RNA using quantitative PCR and a complete set of gene-specific primers corresponding to the stevor repertoire of the parent NF54 isolate. This allowed us to observe that, during prolonged cultivation, the B3B1 clone switched from a state of no detectable stevor expression to expression of several stevor genes (Figure 5E). This change in stevor expression was coupled with an increase in erythrocyte rigidity as measured both by increased retention in the microsphere matrices as well as decreased elongation indices in LORCA experiments (Figure 5A-D). Deformability experiments were assayed at 34 hpi, during the trophozoite stage, when STEVOR proteins are expressed. Differences in retention cannot be attributed to changes in PfEMP3 or KAHRP expression because, both genes are deleted from B3 and its subclone B3B1. In addition, the changes in B3B1 stevor expression were not the result cross-contamination by other cultured parasite lines because its expression pattern was distinct from stevor expression in any of our other cultured clonal lines.
Switching of stevor expression in B3B1 results in increased erythrocyte rigidity. (A,C) Retention rate of erythrocytes infected with B3 (gray bar) and B3B1 before (B3B1 OFF, red bar) or after (B3B1 ON, green bar) switching in stevor gene family expression. Measurements were performed on 2% hematocrit cultures containing 2% to 10% tightly synchronized parasites at 34 hpi. (B,D) LORCA rigidity measurements of erythrocytes infected with B3 (gray bar) and B3B1 before (B3B1 OFF, red bar) or after (B3B1 ON, green bar) switching in stevor gene family expression. Measurements were performed on MACS-concentrated cultures containing 50% synchronized parasites at 34 hpi. (E) Analysis of transcriptional levels for the stevor gene family in B3B1 line before (B3B1 OFF, red bars) and after (B3B1 ON, green bars) switching in expression. Transcriptional analysis was performed at 26 to 28 hpi during the same life cycle as the LORCA and microsphere matrix measurements. Transcription levels were normalized to the transcription level of the seryltRNA synthetase housekeeping gene (PF07_0073).
Switching of stevor expression in B3B1 results in increased erythrocyte rigidity. (A,C) Retention rate of erythrocytes infected with B3 (gray bar) and B3B1 before (B3B1 OFF, red bar) or after (B3B1 ON, green bar) switching in stevor gene family expression. Measurements were performed on 2% hematocrit cultures containing 2% to 10% tightly synchronized parasites at 34 hpi. (B,D) LORCA rigidity measurements of erythrocytes infected with B3 (gray bar) and B3B1 before (B3B1 OFF, red bar) or after (B3B1 ON, green bar) switching in stevor gene family expression. Measurements were performed on MACS-concentrated cultures containing 50% synchronized parasites at 34 hpi. (E) Analysis of transcriptional levels for the stevor gene family in B3B1 line before (B3B1 OFF, red bars) and after (B3B1 ON, green bars) switching in expression. Transcriptional analysis was performed at 26 to 28 hpi during the same life cycle as the LORCA and microsphere matrix measurements. Transcription levels were normalized to the transcription level of the seryltRNA synthetase housekeeping gene (PF07_0073).
Discussion
In the present study, we show that the expression of STEVOR proteins contributes to the rigidity of the infected erythrocyte. As a measure of erythrocyte deformability, we assayed flow versus retention of infected erythrocytes through a matrix of microspheres and LORCA.21 Our initial observations were that 2 parasite clonal lines that vary in their endogenous levels of stevor expression elicit significant differences in erythrocyte deformability at the 34-hpi trophozoite stage, approximately 8 to 12 hours after the beginning of stevor expression. This led us to test the hypothesis that STEVOR proteins are involved in erythrocyte rigidity. This model is qualified by the fact that STEVOR proteins are unlikely to be solely responsible for the induction of erythrocyte membrane rigidity because an increase in erythrocyte rigidity is initially seen by 10 to 22 hpi, before the onset of stevor expression. Additional parasite-encoded proteins probably participate in the induction of erythrocyte rigidity, with RESA being a notable example.7 It is not known whether the other members of the 2TM superfamily (ie, RIFIN and Pfmc-2TM proteins) can complement the biologic functions of STEVOR proteins. Preliminary data suggest that RIFIN, but not Pfmc-2TM, proteins also affect erythrocyte membrane deformability (supplemental Figure 2; and unpublished results). Because rifin expression starts as early as 12 hpi,25 RIFIN proteins may be partially responsible for the rigidity increase observed before 22 hpi, before the transcription of stevor genes. Using the microspheres assay, loss of retention phenotype was observed in early parasite stages of the SB3 line, in which stevor genes are down-regulated, and this might be explained by an extended cross–down-regulation of the rifin gene family expression in this clone.24 A recent study reported that STEVOR proteins are also expressed in merozoites and could be carried into the erythrocyte during invasion, leading to persistence of STEVOR proteins in early stages of parasite development, as detected by Western blot analysis.18 It would be of interest to determine whether STEVOR proteins are localized at the ring-infected erythrocyte membrane and affect its deformability. RESA has been shown to contribute to the parasite-induced erythrocyte rigidity observed before 22 hpi, via interaction with the erythrocyte membrane skeleton. Although RESA plays a significant role in reducing deformability of the infected erythrocyte at the early ring stages of parasite development, it is not expressed during the later stages and thus does not contribute to deformability of mature trophozoite stages.7 Moreover, other exported proteins have been reported to contribute to the overall rigidity of the infected erythrocyte, such as KAHRP or PfEMP3, which both interact with the erythrocyte membrane skeleton.4,26,27 However, the strong increase in rigidity during the life cycle of the KAHRP- and PfEMP3-deficient B3 clone reported here suggests that these proteins play a secondary role in the rigidification of the infected erythrocyte.
Three features are dominantly responsible for erythrocyte rigidity: deformability of the erythrocyte membrane, geometry of the cell determined by the cell surface area-to-volume ratio, and cytoplasmic viscosity determined by intracellular hemoglobin concentration.2 The erythrocyte membrane owes its remarkable deformability to the interactions of the elastic network of skeletal proteins with different integral membrane proteins, such as the interaction of ankyrin and spectrin with the anion transporter Band 3.28,29 The preservation of this architecture aids in the maintenance of membrane deformability in uninfected erythrocytes, whereas the abnormal molecular interactions occurring in P falciparum–infected erythrocytes result in cross-linking of cytoskeletal proteins that can markedly decrease the deformability of the cell.4,30,–32 Correlation of STEVOR expression with erythrocyte rigidity suggests a molecular mechanism, thus far elusive. The topology of STEVOR proteins at the erythrocyte membrane remains controversial, and studies propose alternative models in which STEVOR proteins possess one versus 2 transmembrane domains, with the STEVOR N-terminal region either facing the erythrocyte cytoplasm or exposed at the extracellular face.13,14,18,33 In either model, however, we can speculate an integral membrane structure and possible interaction with erythrocyte cytoskeletal proteins mediated by either the C-terminal, N-terminal, or both domains. For example, induction of spectrin cross-linking has been proposed for the erythrocyte cytoplasm targeted proteins, KAHRP and PfEMP3.4,27,32
Our present data show that STEVOR proteins contribute to the rigidity of the infected erythrocyte, although it is not clear whether these modifications of membrane rheologic properties are essential for the parasite development or are an indirect consequence of STEVOR export at the membrane. Beyond the crucial link between the expression of STEVOR proteins and a cellular phenotype, our results portend important potential consequences in malaria pathogenesis and parasite survival. Cytoadherence of infected erythrocytes in microvessels (arterioles, capillaries, and venules) is essentially PfEMP1-mediated, but STEVOR-associated increased stiffness of infected erythrocytes may further enhance their sequestration. The main expected impact of these processes is an increase in disease severity, as impaired microcirculation is a central mechanism of cerebral malaria and death. Whether they confer a selective advantage to the parasite will depend on a fine balance between the facilitated parasite multiplication induced by sequestration and the increased risk of premature death of the host. In contrast, in patients possessing polymorphic erythrocytes that correlate with decreased malaria severity, such as hemoglobin C patients, the impaired cytoadherence may be linked with changes in erythrocyte deformability, which could further impair deep tissue sequestration and thereby aid in reducing disease burden.34,35
STEVOR proteins are the only variant antigens that have been shown to be localized to the gametocyte-infected erythrocyte membrane, and the same stevor variants are transcribed in gametocytes and their asexual progenitors.15,36 Furthermore, STEVOR expression can be undetectable in some clonal lines in vitro, when gametocytogenesis is dispensable, whereas it is detected at a high level in parasites recently isolated from patients, when gametocytogenesis is critical for parasite transmission.5,16,37 It would be of interest to determine whether STEVOR proteins also impact deformability of erythrocytes hosting gametocytes and whether a potential STEVOR-mediated rigidity may facilitate gametocyte sequestration.
In conclusion, published work by our and other laboratories suggests that STEVOR protein function is integral within the erythrocyte membrane, and stevor family members are subject to switching in gene expression profiles that might underpin antigenic variation at the erythrocyte surface. In our present study, we show that expression of STEVOR proteins is correlated with increased erythrocyte membrane rigidity; thus, STEVOR proteins might play a role in facilitating parasite sequestration in deep tissue vasculature. Such a role for STEVOR proteins might be critical in vivo but dispensable in cultured parasite lines.
This article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Geneviève Milon and Narla Mohandas for fruitful discussions and for benefiting from the National Institutes of Health Project Mechanisms of Erythrocytic Infection and Anemia in Malaria (award no. 5P01HL078826-06).
This work was supported by François Lacoste (Fondation Ackerman-Fondation de France) and the Fondation Symphasis. T.J.T. was supported by the National Institutes of Health/National Institute of Allergy and Infectious Diseases (grant 1R01AI080754-01A1) and the William Randolph Hearst Foundation.
National Institutes of Health
Authorship
Contribution: S.S., S.E., G.B., S.P., I.S., and C.L. performed experiments, analyzed results, and produced the figures; E.B. performed statistical analysis; P.B., K.W.D., O.M.-P., and P.H.D. analyzed data and contributed vital new reagents or analytical tools; and S.S., T.J.T., and C.L. designed the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas J. Templeton, Department of Microbiology and Immunology, Weill Cornell Medical College, and the Weill Graduate School of Medical Sciences of Cornell University, 1300 York Ave, New York, NY 10021; e-mail: tjt2001@med.cornell.edu; and Catherine Lavazec, Institut Pasteur, Unité d'Immunologie Moléculaire des Parasites, Département de Parasitologie Mycologie, CNRS, URA 2581, Paris, France; e-mail: clavazec@pasteur.fr.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal