While testing the effect of the (β15-66)2 fragment, which mimics a pair of fibrin βN-domains, on the morphology of endothelial cells, we found that this fragment induces redistribution of vascular endothelial–cadherin in a process that is inhibited by the receptor-associated protein (RAP). Based on this finding, we hypothesized that fibrin may interact with members of RAP-dependent low-density lipoprotein (LDL) receptor family. To test this hypothesis, we examined the interaction of (β15-66)2, fibrin, and several fibrin-derived fragments with 2 members of this family by ELISA and surface plasmon resonance. The experiments showed that very LDL (VLDL) receptor (VLDLR) interacts with high affinity with fibrin through its βN-domains, and this interaction is inhibited by RAP and (β15-66)2. Furthermore, RAP inhibited transendothelial migration of neutrophils induced by fibrin-derived NDSK-II fragment containing βN-domains, suggesting the involvement of VLDLR in fibrin-dependent leukocyte transmigration. Our experiments with VLDLR-deficient mice confirmed this suggestion by showing that, in contrast to wild-type mice, fibrin-dependent leukocyte transmigration does not occur in such mice. Altogether, the present study identified VLDLR as a novel endothelial cell receptor for fibrin that promotes fibrin-dependent leukocyte transmigration and thereby inflammation. Establishing the molecular mechanism underlying this interaction may result in the development of novel inhibitors of fibrin-dependent inflammation.
Introduction
Thrombin-mediated conversion of fibrinogen into fibrin plays a prominent role in preventing the loss of blood on vascular injury. During this conversion, thrombin removes from fibrinogen 2 pairs of N-terminal fibrinopeptides, fibrinopeptide A (FpA) and fibrinopeptide B (FpB), enabling spontaneous polymerization of fibrin monomers into an insoluble fibrin clot. The clot seals the injured vasculature and serves as a provisional matrix that participates in subsequent wound healing and other important physiologic and pathologic processes through the interaction with various plasma proteins and cell types. Specifically, the interaction of fibrin with endothelial cells contributes to anchoring of the clot to places of vascular injury and then promotes transendothelial migration of leukocytes and thereby inflammation and capillary tube formation, that is, angiogenesis.1,,–4
Several receptors on the endothelial cell surface mediate the interaction of fibrin(ogen) with endothelial cells. They include integrins αVβ3, αVβ5, and α5β1,5,–7 and nonintegrin receptors ICAM-1, vascular endothelial (VE)–cadherin, and proteoglycans.8,–10 The interaction of endothelial cell integrins with fibrin-(ogen) occurs mainly through the fibrin(ogen) RGD sequences and promotes endothelial cell adhesion and spreading.5,7 This interaction may also promote cell migration and angiogenesis.3,7 The interaction with proteoglycans mediates binding of fibrin(ogen) to endothelial cells,10 whereas that with ICAM-1 promotes adhesion and transendothelial migration of leukocytes.2,8 Leukocyte transmigration is also supported by the interaction of fibrin or its degradation products with VE-cadherin4,11 ; furthermore, this interaction was shown to promote fibrin-dependent angiogenesis.9,12
Fibrinogen is a complex multidomain protein whose multiple functions are carried out through the interaction of its individual domains with various proteins and cell types. Several fibrin (ogen) domains are involved in the interaction with endothelial cell receptors. Among them, 2 βN-domains, each formed by the N-terminal portion of the fibrin β chain, were shown to be responsible for the interaction with VE-cadherin.13,14 Previous studies have reported that the β15-42 sequence, representing approximately one-half of the fibrin βN-domain, is involved in the interaction with VE-cadherin, and the exposure of this sequence after the removal of FpB (residues Bβ1-14) from fibrinogen is necessary for fibrin-dependent capillary tube formation.1,9,12 Another study found that N-terminal disulfide knot II (NDSK-II) fragment, which corresponds to the central region of fibrin and contains the β15-42 sequence, induces leukocyte transmigration in vitro, and a synthetic peptide β15-42 corresponding to this sequence inhibits this process.11 To explain this finding, it was hypothesized that NDSK-II induces this process by bridging inflammatory cells to the endothelium through the interaction with CD11c receptor of the former and VE-cadherin of the latter; therefore, the inhibitory effect of β15-42 was associated with its ability to compete with NDSK-II for the interaction with VE-cadherin.4,11 Our recent finding that dimeric β15-42 containing fragments inhibited infiltration of leukocytes into the peritoneum in a mouse model of peritonitis by > 50%15 highlights the importance of this mechanism in the overall transmigration of leukocytes during inflammation.
Although the hypothesis mentioned in the preceding paragraph coherently explains the inhibitory effect of the β15-42 peptide and its anti-inflammatory properties, it does not take into account that the affinity of this peptide to VE-cadherin is very low. Our previous study reported that even at the concentration of 1μM this peptide did not exhibit any binding to VE-cadherin, and only a dimeric fragment, (β15-66)2, corresponding to the full-length βN-domain and mimicking its dimeric arrangement in fibrin bound VE-cadherin with high affinity.13 Our recent study confirmed that the affinity of the β15-42 peptide to VE-cadherin is very low (Kd = 267μM).15 At the same time, the affinity of this peptide to endothelial cell surface was found to be 3 orders of magnitude higher (Kd = 0.18μM).12,16 On the basis of these findings, we hypothesized that the β15-42 peptide and fibrin may interact with other endothelial cell receptors and that such interaction(s) may contribute to the anti-inflammatory effect of the former and proinflammatory effect of the latter.15
In the present study, we found that fibrin interacts with endothelial cells through their very low-density lipoprotein (VLDL) receptor (VLDLR) and localized VLDLR-binding site in fibrin βN-domains. Our in vitro and in vivo experiments showed that this interaction promotes fibrin-dependent transendothelial migration of leukocytes. Thus, we identified VLDLR as a novel endothelial cell receptor for fibrin that modulates fibrin-dependent leukocyte transmigration and thereby inflammation.
Methods
Proteins, Abs, and reagents
Human fibrinogen (plasminogen-, VWF-, and fibronectin-depleted) was from Enzyme Research Laboratories. The soluble form of human VLDLR that contains the entire ectodomain (sVLDLR) was prepared with the Drosophila Expression System (Invitrogen) and purified as previously described.17 LDLR-related protein (LRP) was isolated from human placenta.18 Human recombinant receptor-associated protein (RAP) was expressed in Escherichia coli and purified as described.19 Phorbol 12-myristate 13-acetate (PMA) was from Promega, N-formyl-Met-Leu-Phe (fMLP), extravidin-alkaline phosphatase, and FITC-labeled phalloidin were from Sigma-Aldrich, Calcein AM fluorescent dye and anti–VE-cadherin mAb 55-7H1 were from BD Biosciences. Alexa 568–conjugated anti–mouse polyclonal Abs and TO-PRO3 were from Invitrogen. Goat secondary anti–mouse polyclonal Abs conjugated with HRP and HRP substrate SureBlue 3–3′-5–5′-tetramethylbenzidine (TMB) were from KPL. Anti-VLDLR mAb 1H5, 5F3, and 1H10 and mAb 5A6 that recognizes the LRP light chain have been described.17,20
Preparation of fibrin(ogen) fragments
The recombinant (β15-66)2 fragment and synthetic (β15-44)2 and scrambled (β15-44)2 fragments were prepared as described earlier.13,15 The recombinant αC-fragment (residues Aα221-610) corresponding to the fibrinogen αC region was produced in E coli.21 The fibrinogen-derived E3 fragment and fibrin-derived D-D:E1 complex, and D-D and E1 fragments were prepared from plasmin digests of fibrinogen and fibrin, respectively.22,23 NDSK fragment corresponding to the central region of fibrinogen was prepared by digestion of human fibrinogen with CNBr.9 NDSK-II fragment lacking FpA and FpB was prepared by incubation of NDSK with thrombin-agarose as was described.15 The purity of all fragments was confirmed by SDS-PAGE. All fragments used in cell and animal experiments were additionally purified on Detoxi-Gel column (Thermo Scientific) to remove endotoxin contamination. Final endotoxin levels determined with Limulus amebocyte lysate test QCL-1000 (Lonza) did not exceed 0.1 EU/mg for all fragments tested.
Mice
Wild-type C57BL/6 mice aged 8-12 weeks were from The Jackson Laboratory. VLDLR-deficient (VLDLR−/−) mice on a mixed background were also obtained from The Jackson Laboratory and bred to 10 generations with a C57BL/6 background. All mice were housed in a pathogen-free facility, and all procedures were performed with approval of the University of Maryland Institutional Animal Care and Use Committee.
Solid-phase binding assay
Wells of Immulon 2HB microtiter plates were coated overnight at 4°C with (β15-66)2 at 2 μg/mL in 0.1M Na2CO3, pH 9.5 (coating buffer). The wells were then blocked with SuperBlock blocking buffer in TBS (20mM tris, pH 7.4, 150mM NaCl; Thermo Scientific) for 1 hour at room temperature. After washing of the wells with TBS containing 0.05% Tween 20 and 1mM CaCl2 (binding buffer), the indicated concentrations of sVLDLR or LRP in this buffer were added to the wells and incubated for 1 hour at 37°C. Bound receptors were detected by reactions with a mixture of anti-VLDLR mAb 1H5, 5F3, and 1H10 or with anti-LRP mAb 5A6 (1 hour at 37°C) and the HRP-conjugated goat anti–mouse polyclonal Abs (1 hour at 37°C). The peroxidase substrate, SureBlue TMB, was added to the wells, and the amount of bound ligand was measured spectrophotometrically at 450 nm. Data were analyzed by nonlinear regression analysis as described previously.14,15 Binding of sVLDLR and LRP to immobilized fibrin and inhibition effect of RAP and (β15-66)2 on this binding was studied essentially as described earlier, except that the wells were blocked with BSA instead of SuperBlock blocking buffer to reduce nonspecific binding. To prepare fibrin surface, wells of Immulon 2HB microtiter plates were coated overnight at 4°C with fibrinogen at 5 μg/mL in the coating buffer and then incubated with 0.1 NIH U/mL thrombin for 1 hour at 37°C to convert fibrinogen into fibrin. In the inhibition experiments, sVLDLR at 10nM in the binding buffer was preincubated with 20nM RAP or 10μM (β15-66)2 for 1 hour at 37°C, and 100-μL aliquots of the mixtures were added to the wells and incubated for 1 hour at 37°C, and bound receptors were detected as described earlier. All experiments were repeated at least twice.
Surface plasmon resonance analysis
Interaction of fibrin(ogen) fragments with immobilized sVLDLR or LRP was studied by surface plasmon resonance (SPR) with the BIAcore 3000 biosensor (BIAcore AB), essentially as described previously.14 Briefly, immobilization of sVLDLR and LRP to the CM5 sensor chip was performed with the amine coupling kit (GE Healthcare) according to the manufacturer's procedure. Binding experiments were performed in binding buffer HBS-P (GE Healthcare) containing 1mM CaCl2. Fibrin(ogen) fragments were injected at the indicated concentrations, and the association/dissociation between them and the immobilized receptors was monitored by the change in the SPR response. To regenerate the chip surface, complete dissociation of the complex was achieved by adding 100mM H3PO4 for 30 seconds followed by re-equilibration with the binding buffer. All experiments were repeated at least twice. Experimental data were analyzed with BIAevaluation 4.1 software supplied with the instrument. The dissociation equilibrium constant, Kd, was calculated as Kd = kdiss/kass, where kass and kdiss represent kinetic constants that were estimated by global analysis of the association/dissociation data with the 1:1 Langmurian interaction model (kinetic analysis). To confirm the kinetic analysis, Kd was also estimated by analysis of the association data with the steady-state affinity model (equilibrium analysis).
Cell culture and treatments
HUVECs, purchased from Lonza, were grown in EGM-2 complete media according to the manufacturer's instruction and used at passage 4-6. The HL-60 promyelocytic cell line (ATCC) was cultured and differentiated to a neutrophil-like lineage as described earlier.15 Differentiated HL-60 cells were labeled with Calcein AM fluorescent dye (BD Biosciences) in serum-free IMDM medium. All cell lines were cultured or incubated at 37°C and 5% CO2.
Immunofluorescence microscopy
HUVECs were grown on gelatin-coated glass coverslips until confluence. After treatments with (β15-66)2 or RAP, the cells were washed with PBS and fixed with solution containing 4% formaldehyde, 5% sucrose in PBS for 20 minutes at room temperature. The fixed cells were permeabilized with 0.4% Triton X-100 for 5 minutes, blocked with 5% goat serum for 1 hour, and then incubated with anti–VE-cadherin mAb 55-7H1 at 6 μg/mL for 3 hours at 37°C. Subsequently, the coverslips were washed with PBS and incubated with secondary Alexa 568–conjugated anti–mouse polyclonal Ab (3 μg/mL; Invitrogen) for 1 hour at 37°C. FITC phalloidin (2 μg/mL) and TO-Pro3 dye (0.5 μg/mL) were included in secondary Ab mixture to stain actin filaments and nuclei. After washing, the coverslips were mounted onto glass slides with FluorSave Reagent (Calbiochem) and viewed with Nikon Eclipse E800 microscope and laser scanning system Radiance 2100 (Bio-Rad/Carl Zeiss MicroImaging GmbH) and using Nikon Plan Apo 60×A/1.40 oil objective. The images were captured using the Radiance 2100 software.
Leukocyte transendothelial migration assay
Transendothelial migration experiments were performed with 24-well plates containing 8-μm pore size PET membrane inserts (BD Biosciences) as described earlier.15 Briefly, HUVECs were seeded onto the insert membrane precoated with gelatin and grown to confluence for 3 days without medium in the lower chamber. Integrity of confluent endothelial monolayers was confirmed by staining the membrane with fixed cells with Coomassie dye. HUVECs were washed twice with IMDM and serum-starved for 2 hours before experiments. Calcein AM-labeled differentiated HL-60 cells were stimulated with 100nM PMA and washed twice with IMDM, and 5 × 105 stimulated cells in IMDM containing 1.5μM NDSK-II with or without 10μM (β15-66)2, 0.5μM RAP were added on top of the HUVEC monolayer. The inserts were placed into the wells each containing 100nM chemoattractant fMLP in IMDM. Transmigration proceeded for 4 hours and was stopped by removing the inserts from the wells. Migrated HL-60 cells were recovered from the bottom of the wells and counted with fluorescence plate reader at 480 nm/530 nm.
Mouse model of peritonitis
Wild-type or VLDLR−/− mice (4-6 per group) were injected intraperitoneally with 3.85% Bacto Fluid thioglycollate (1 mL per mouse) to induce leukocyte infiltration into the peritoneum. To test the effect of the (β15-66)2 or (β15-44)2 fragments on leukocyte infiltration in wild-type and VLDLR−/− groups, mice received an intravenous injection (via the tail vein) of the fragments, both at 80μM in 200 μL of PBS (Lonza), before intraperitoneal injection of thioglycollate. Mice in control groups received an intravenous injection of the same volume of PBS or scrambled (β15-44)2 at 80μM. Four hours after the injections, mice in each group were killed by CO2 inhalation followed by cervical dislocation and injected intraperitoneally with 3 mL of ice-cold PBS, and total lavage fluid was withdrawn after massaging their abdomens. The total leukocyte (neutrophil) number in the peritoneal lavage was determined with a hemocytometer.
Statistical analysis
Statistical analysis was performed using the Student t test with a P value < .05 being considered significant. All statistical analyses were performed in SigmaPlot 8.0 software (Systat Software).
Results
Effect of the (β15-66)2 fragment on endothelial cells
Previous studies have reported that the recombinant (β15-66)2 fragment, which mimics the structure and functional properties of fibrin βN-domains, interacts with endothelial cell receptor VE-cadherin.13,15 To test the effect of this fragment on the morphology of endothelial cells in culture, we used confocal fluorescence microscopy. HUVECs were grown on gelatin-coated glass coverslips to confluence and then incubated with 250nM (β15-66)2 for 6 hours. The cells were fixed, permeabilized, and immunostained with the mAb 55-7H1 and FITC-labeled phalloidin to detect VE-cadherin and actin filaments, respectively, and with TO-PRO3 to visualize nuclei. Visual comparison of the images presented in Figure 1A through F showed that, although actin cytoskeleton in both fragment-treated and control cells has not been changed (Figure 1B,E), cell junctions formed by VE-cadherin were tight and sharp in control HUVECs but appear rough and loose in the fragment-treated cells (Figure 1A,D). Furthermore, although there was very little intracellular VE-cadherin staining in control cells, we observed significant amounts of intracellular VE-cadherin concentrated in perinuclear area of cells treated with (β15-66)2 (Figure 1C,F). These results suggest that treatment of endothelial cells with the (β15-66)2 fragment resulted in redistribution of VE-cadherin between the cell surface and the cytosol and loosening of cell–cell junctions.
Effect of the (β15-66)2 fragment on the distribution of VE-cadherin in endothelial cells. (A-F) HUVECs grown on glass coverslips to confluence were incubated for 6 hours without (A-C) or with 250nM (β15-66)2 fragment (D-F), fixed, permeabilized, and immunostained for VE-cadherin (red in panels A and D), actin (green in panels B and E), and nuclei (blue in panels C and F). (G-I) The inhibitory effect of RAP on the (β15-66)2–induced redistribution of VE-cadherin in HUVECs is shown. Cells grown to confluence were incubated for 24 hours with media alone (G), with 250nM (β15-66)2 (H), or with 250nM (β15-66)2 plus 500nM RAP (I), fixed, permeabilized, and immunostained for VE-cadherin. All confocal images were taken through the middle of the cell layer. Magnification ×63, oil immersion.
Effect of the (β15-66)2 fragment on the distribution of VE-cadherin in endothelial cells. (A-F) HUVECs grown on glass coverslips to confluence were incubated for 6 hours without (A-C) or with 250nM (β15-66)2 fragment (D-F), fixed, permeabilized, and immunostained for VE-cadherin (red in panels A and D), actin (green in panels B and E), and nuclei (blue in panels C and F). (G-I) The inhibitory effect of RAP on the (β15-66)2–induced redistribution of VE-cadherin in HUVECs is shown. Cells grown to confluence were incubated for 24 hours with media alone (G), with 250nM (β15-66)2 (H), or with 250nM (β15-66)2 plus 500nM RAP (I), fixed, permeabilized, and immunostained for VE-cadherin. All confocal images were taken through the middle of the cell layer. Magnification ×63, oil immersion.
Effect of RAP on the (β15-66)2–induced redistribution of VE-cadherin in endothelial cells
The observed redistribution of VE-cadherin may be connected with its internalization, although new synthesis of VE-cadherin, inhibition of its transit to the surface, or some other reasons cannot be excluded. Because the LDLR family members are known to bind and internalize a broad spectrum of various structurally unrelated ligands,24 one can expect them to be involved in such redistribution/internalization. To test this speculation, we studied the effect of RAP, a well-known inhibitor of the LDLR family members19,25 on the (β15-66)2–induced redistribution of VE-cadherin in HUVECs in the experiments similar to those described earlier. HUVECs grown to confluence were incubated with 250nM (β15-66)2 for 24 hours in the presence or absence of 500nM RAP, then fixed and immunostained with mAb 55-7H1 to detect VE-cadherin distribution by confocal microscopy. The results presented in Figure 1G and H show that treatment of HUVECs with (β15-66)2 resulted in a significant increase in intracellular VE-cadherin. Note that in this case the (β15-66)2–induced changes in the morphology of HUVECs were more profound than those shown in Figure 1A and D, most probably because of the longer incubation time. This effect was totally abrogated by RAP (Figure 1I), suggesting the involvement of a RAP-sensitive receptor(s) in this process. To test this suggestion, we next studied interaction of the (β15-66)2 fragment with 2 members of the LDLR family, the VLDLR and LRP.
Evidence for the interaction of fibrin βN-domains with VLDLR
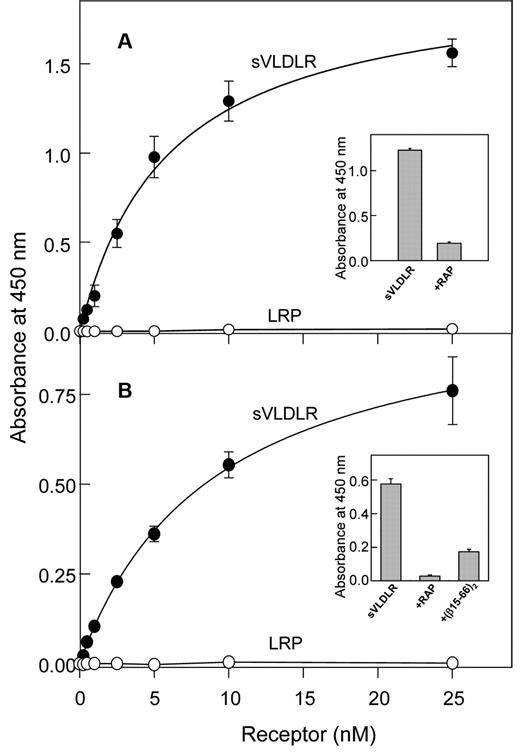
To determine whether the VLDLR and/or LRP interact with (β15-66)2, we performed an ELISA. In this assay, increasing concentrations of the sVLDLR or LRP were incubated with the immobilized (β15-66)2 fragment, and bound receptor was detected with the cocktail of specific anti-VLDLR mAbs (mAb 1H5, mAb 5F3, and mAb 1H10) or anti-LRP mAb 5A6, respectively. The results indicated prominent binding of sVLDLR to immobilized (β15-66)2 whereas LRP exhibited no binding (Figure 2A). Note that both sVLDLR and LRP used in these experiments were functionally active because in a control experiment they both bound RAP (not shown). The binding of sVLDLR to immobilized (β15-66)2 was dose dependent, and the Kd value determined for this binding was found to be 5.7 ± 0.4nM (n = 2). The binding was specific because in the presence of RAP the interaction of sVLDLR with (β15-66)2 was substantially reduced (Figure 2A inset). Similarly, sVLDLR bound to immobilized fibrin with Kd = 9.2 ± 0.5nM (n = 2; Figure 2B), and this binding was significantly inhibited by RAP and the (β15-66)2 fragment (Figure 2B inset). These experiments clearly indicate that the VLDLR interacted with fibrin and its βN-domains with high affinity and that this interaction was specific.
Analysis of interaction of the (β15-66)2 fragment and fibrin with the LDLR family members, VLDLR and LRP, by ELISA. Increasing concentrations of sVLDLR (●) or LRP (○) were incubated with the microtiter wells coated with the (β15-66)2 fragment (A) or fibrin (B), and the bound receptors were detected with the specific mAbs as described in “Solid-phage binding assay.” The curves are representative of 2 independent experiments; error bars represent the SD of triplicate determinations. Binding of sVLDLR at 10nM to immobilized (β15-66)2 in the absence and presence of 20nM RAP is shown in panel A inset. Binding of sVLDLR at 10nM to immobilized fibrin in the absence and presence of 20nM RAP or 10μM (β15-66)2 is shown in panel B inset.
Analysis of interaction of the (β15-66)2 fragment and fibrin with the LDLR family members, VLDLR and LRP, by ELISA. Increasing concentrations of sVLDLR (●) or LRP (○) were incubated with the microtiter wells coated with the (β15-66)2 fragment (A) or fibrin (B), and the bound receptors were detected with the specific mAbs as described in “Solid-phage binding assay.” The curves are representative of 2 independent experiments; error bars represent the SD of triplicate determinations. Binding of sVLDLR at 10nM to immobilized (β15-66)2 in the absence and presence of 20nM RAP is shown in panel A inset. Binding of sVLDLR at 10nM to immobilized fibrin in the absence and presence of 20nM RAP or 10μM (β15-66)2 is shown in panel B inset.
This finding was confirmed by SPR experiments, in which, in contrast to the ELISA experiments, VLDLR and LRP were immobilized on the surface of a sensor chip while their binding partners were in solution. First, when (β15-66)2 at 1μM was added to immobilized sVLDLR or LRP, a prominent binding was observed only to sVLDLR (Figure 3A). Second, when increasing concentrations of (β15-66)2 were added to immobilized sVLDLR, the binding of this fragment was dose dependent (Figure 3B), and the Kd determined by the kinetic analysis (see “Surface plasmon resonance analysis”) was found to be 3.6 ± 0.9nM (n = 4), that is, very close to that determined by ELISA. Finally, when (β15-66)2 and RAP were injected individually at 500nM and 50nM, respectively, they both exhibited prominent binding to immobilized sVLDLR; however, when they were injected simultaneously at the same molar concentrations, the SPR signal was almost the same as that for RAP alone (Figure 4A), indicating that RAP inhibited the interaction of (β15-66)2 with sVLDLR. In agreement, when a mixture of RAP and (β15-66)2 was injected after RAP or after (β15-66)2, the SPR signal was also almost the same as that for RAP alone (Figure 4B). Altogether, these experiments indicate that the (β15-66)2 fragment bound to the VLDLR with high affinity and the binding was highly specific.
Analysis of interaction of the (β15-66)2 fragment with the LDLR family members, VLDLR and LRP, by SPR. (A) The (β15-66)2 fragment at 1μM was added to immobilized sVLDLR or LRP, and its association/dissociation was monitored in real time. (B) The (β15-66)2 fragment at increasing concentrations, 0.5, 1, 2.5, 5, and 10nM, was added to immobilized sVLDLR, and its association/dissociation was monitored in real time (solid curves). The dotted curves represent the best fit of the data with the use of the kinetic analysis. The curves in panels A and B are representative of 2 and 4 independent experiments, respectively. All experiments were performed in HBS-P buffer containing 1mM CaCl2.
Analysis of interaction of the (β15-66)2 fragment with the LDLR family members, VLDLR and LRP, by SPR. (A) The (β15-66)2 fragment at 1μM was added to immobilized sVLDLR or LRP, and its association/dissociation was monitored in real time. (B) The (β15-66)2 fragment at increasing concentrations, 0.5, 1, 2.5, 5, and 10nM, was added to immobilized sVLDLR, and its association/dissociation was monitored in real time (solid curves). The dotted curves represent the best fit of the data with the use of the kinetic analysis. The curves in panels A and B are representative of 2 and 4 independent experiments, respectively. All experiments were performed in HBS-P buffer containing 1mM CaCl2.
Inhibitory effect of RAP on the interaction of the (β15-66)2 fragment with the VLDLR detected by SPR. (A) RAP (50nM; solid line), 500nM (β15-66)2 (dotted line), or their mixture [50nM RAP + 500nM (β15-66)2] (broken line), were added to immobilized VLDLR and their association/dissociation was monitored in real time. (B) The same mixture of RAP and (β15-66)2 was added after the addition of 50nM RAP (solid line) or 500nM (β15-66)2, (broken line); arrows indicate injection of the mixture. The curves are representative of 2 independent experiments.
Inhibitory effect of RAP on the interaction of the (β15-66)2 fragment with the VLDLR detected by SPR. (A) RAP (50nM; solid line), 500nM (β15-66)2 (dotted line), or their mixture [50nM RAP + 500nM (β15-66)2] (broken line), were added to immobilized VLDLR and their association/dissociation was monitored in real time. (B) The same mixture of RAP and (β15-66)2 was added after the addition of 50nM RAP (solid line) or 500nM (β15-66)2, (broken line); arrows indicate injection of the mixture. The curves are representative of 2 independent experiments.
Testing the interaction of other fibrin(ogen) fragments with the VLDLR
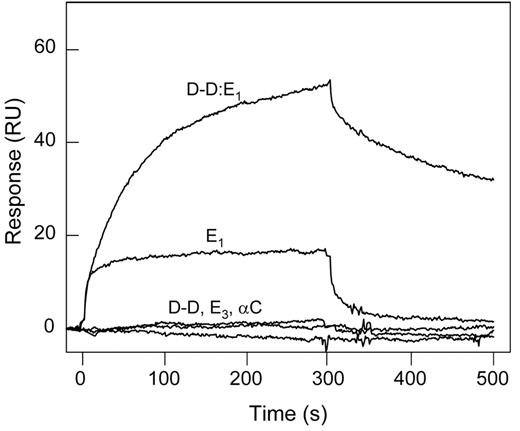
The (β15-66)2 fragment corresponds to the fibrin βN-domains, representing only a small portion of the molecule. To test if other fibrin regions/domains are involved in the interaction with the VLDLR, we studied binding of the latter with the proteolytically prepared fibrin fragments, D-D and E1, and the recombinant Aα221-610 fragment corresponding to the fibrin(ogen) αC regions. These fragments together cover practically the whole fibrin molecule. In SPR experiments, when these fragments, each at 1μM, were added to the immobilized VLDLR, only E1 exhibited a prominent binding, whereas D-D and Aα221-610 failed to bind (Figure 5). In addition, the E3 fragment devoid of the βN-domains23 exhibited no interaction with sVLDLR. In another experiment, when the D-D:E1 complex prepared from the early digest of fibrin22 and mimicking the arrangement and interactions of the D and E regions in the latter was added to the immobilized VLDLR, it also exhibited a prominent binding, most probably through its E1 component (Figure 5). Note that the same fragments failed to bind to immobilized LRP in similar experiments (not shown), further confirming that fibrin does not interact with this receptor. Altogether, these experiments indicate that the βN-domains are the only structures in fibrin that interact with the VLDLR. They also suggest that these domains are reactive in fibrin.
Interaction of fibrin(ogen) fragments with the VLDLR detected by SPR. Fibrin(ogen) fragments, D-D, E1, E3, αC fragment, or the (D-D)E1 complex, all at 1μM, were added to immobilized sVLDLR, and their association/dissociation was monitored in real time. The curves are representative of 2 independent experiments performed in HBS-P buffer containing 1mM CaCl2.
Interaction of fibrin(ogen) fragments with the VLDLR detected by SPR. Fibrin(ogen) fragments, D-D, E1, E3, αC fragment, or the (D-D)E1 complex, all at 1μM, were added to immobilized sVLDLR, and their association/dissociation was monitored in real time. The curves are representative of 2 independent experiments performed in HBS-P buffer containing 1mM CaCl2.
Effect of RAP on transendothelial migration of leukocytes in vitro
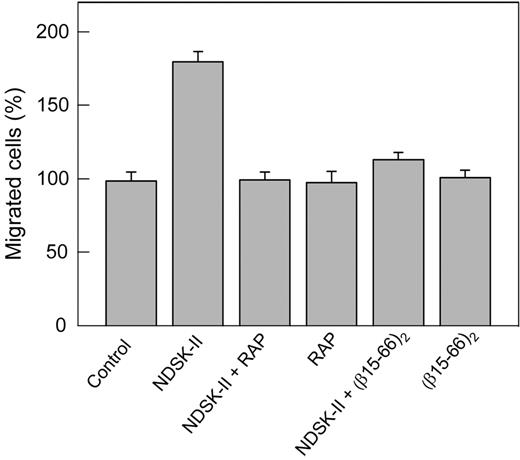
The interaction of NDSK-II, a fibrin fragment containing a pair of the βN-domains, with VE-cadherin has been suggested to promote transendothelial migration of leukocytes, and the β15-42 fragment derived from these domains has been shown to inhibit this process.11 Our recent study confirmed this finding and showed that the dimeric version of β15-42, the (β15-44)2 fragment, as well as (β15-66)2 dimer, are even more potent inhibitors of this process.15 Furthermore, the data presented in this study indicate that the (β15-66)2 fragment caused loosening and even disruption of cell–cell junctions in HUVECs (Figure 1D,H), which, in principle, may affect leukocyte transmigration. Because this effect was inhibited by RAP, we reasoned that RAP may also inhibit leukocyte transmigration. To test this hypothesis, we used an in vitro transendothelial leukocyte migration assay, in which transmigration of DMSO-induced HL-60 cells mimicking neutrophils26 through HUVEC monolayer was induced by NDSK-II, corresponding to the central region of fibrin. Note that such cells were shown to constitute a valid model system for the analysis of human neutrophil migration.26 The results presented in Figure 6 indicate that NDSK-II promoted transendothelial migration of these cells, as was expected on the basis of the previous results.11,15 However, in the presence of RAP, this effect was completely abrogated. In control experiments, RAP itself neither increased nor decreased the transmigration, and the (β15-66)2 fragment inhibited the NDSK-II–induced transmigration practically to the same degree as RAP. Altogether, these experiments indicate that RAP inhibits transendothelial migration of leukocytes induced by NDSK-II, suggesting the involvement of the VLDLR in this process.
Inhibitory effect of RAP on NDSK-II–induced transendothelial migration of neutrophils in vitro. HUVECs were grown to confluence on gelatin-coated cell culture inserts. Calcein AM–labeled HL-60 cells differentiated into neutrophil-like cells were added to the upper chambers on top of the HUVEC monolayer in the presence of PBS used as a control or 1.5μM NDSK-II without or with 0.5μM RAP or 10μM (β15-66)2; RAP and (β15-66)2 at the same concentrations without NDSK-II were also added. The cells were allowed to migrate into the lower chambers containing chemoattractant fMLP for 4 hours at 37°C, collected, and measured by fluorescence at 530 nm. The results are expressed as percentage of HL-60 cells migrated in the presence of PBS (control). The graph shows combined data obtained from 2 independent experiments; bars denote means ± SEs (n = 6-9 wells for each condition).
Inhibitory effect of RAP on NDSK-II–induced transendothelial migration of neutrophils in vitro. HUVECs were grown to confluence on gelatin-coated cell culture inserts. Calcein AM–labeled HL-60 cells differentiated into neutrophil-like cells were added to the upper chambers on top of the HUVEC monolayer in the presence of PBS used as a control or 1.5μM NDSK-II without or with 0.5μM RAP or 10μM (β15-66)2; RAP and (β15-66)2 at the same concentrations without NDSK-II were also added. The cells were allowed to migrate into the lower chambers containing chemoattractant fMLP for 4 hours at 37°C, collected, and measured by fluorescence at 530 nm. The results are expressed as percentage of HL-60 cells migrated in the presence of PBS (control). The graph shows combined data obtained from 2 independent experiments; bars denote means ± SEs (n = 6-9 wells for each condition).
Evidence for the involvement of the VLDLR in fibrin-dependent leukocyte transmigration in vivo
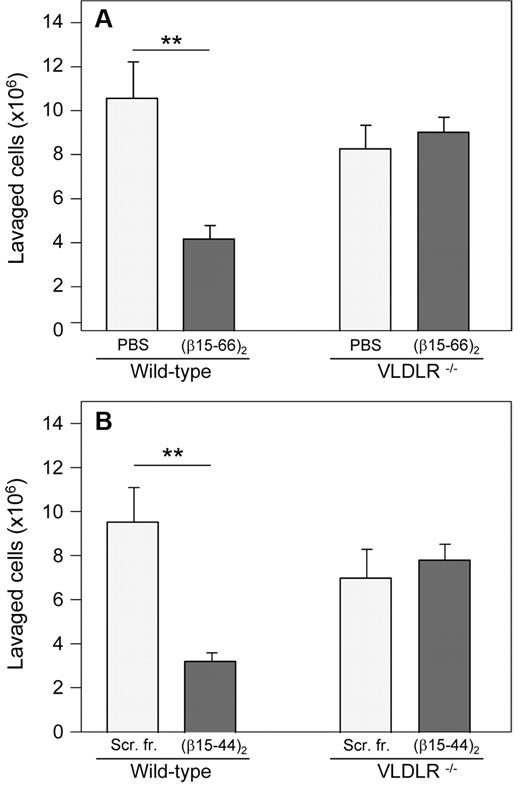
To investigate the involvement of the VLDLR receptor in leukocyte transmigration in vivo, we studied this process in VLDLR−/− mice. With the use of the in vivo peritonitis model, we compared infiltration of leukocytes (neutrophils) from the circulation into the peritoneum in wild-type and VLDLR−/− mice. The experiment showed that leukocyte transmigration in VLDLR−/− mice was moderately (by ∼ 22%) lower than that in wild-type mice (Figure 7A), although the difference was not statistically significant. When both wild-type and VLDLR−/− mice were injected intravenously either with PBS (control mice) or with the (β15-66)2 fragment before intraperitoneal injection of thioglycollate, this fragment inhibited leukocyte transmigration only in wild-type mice; no effect was observed in VLDLR−/− mice. A very similar effect was observed in another experiment, in which wild-type and VLDLR−/− mice were injected with the shorter (β15-44)2 dimer, which in our previous study15 exhibited similar anti-inflammatory effect as the (β15-66)2 fragment, and control mice were injected with scrambled (β15-44)2 instead of PBS (Figure 7B). These results indicate that fibrin-dependent leukocyte transmigration, which in wild-type mice is inhibited by (β15-66)2 or (β15-44)2, does not occur in mice lacking VLDLR. They directly confirm the involvement of the VLDLR in fibrin-dependent leukocyte transmigration. Moreover, they suggest that VLDLR is absolutely necessary for this process.
Inhibitory effect of β15-42–containing dimeric fibrin fragments on neutrophil infiltration in vivo in a mouse model of peritonitis. The recombinant (β15-66)2 fragment (A) or the synthetic (β15-44)2 fragment (B), each in 200 μL of PBS at 80μM (total dose per mouse was 16 nmol), was injected intravenously in wild-type and VLDLR−/− mice; control mice were injected with 200 μL of PBS (A) or scrambled (β15-44)2 fragment (Scr. fr.) at 80μM in 200 μL of PBS (B). The number of infiltrated leukocytes (neutrophils) was estimated as described in the “Leukocyte transendothelial migration assay.” The results are means ± SEs (n = 4, A; n = 6, B). **P < .01.
Inhibitory effect of β15-42–containing dimeric fibrin fragments on neutrophil infiltration in vivo in a mouse model of peritonitis. The recombinant (β15-66)2 fragment (A) or the synthetic (β15-44)2 fragment (B), each in 200 μL of PBS at 80μM (total dose per mouse was 16 nmol), was injected intravenously in wild-type and VLDLR−/− mice; control mice were injected with 200 μL of PBS (A) or scrambled (β15-44)2 fragment (Scr. fr.) at 80μM in 200 μL of PBS (B). The number of infiltrated leukocytes (neutrophils) was estimated as described in the “Leukocyte transendothelial migration assay.” The results are means ± SEs (n = 4, A; n = 6, B). **P < .01.
Discussion
Our observation that the (β15-66)2 fragment, which mimics the fibrin βN-domains, alters the morphology of HUVEC adherens junctions formed by VE-cadherin making them more loose (Figure 1) suggests that the interaction of this fragment with VE-cadherin could result in opening of these junctions and thereby promoting transendothelial migration of leukocytes. This finding is in agreement with the previous observations that fibrinogen or its β15-42 peptide increases permeability of HUVEC monolayer and that fibrinogen induces intracellular gap formation and cytosolic relocation of VE-cadherin through the interaction of its β15-42 sequence with VE-cadherin.27 In contrast, a recent in vivo study that used 2 shock models reported that β15-42 preserves endothelial barrier function by inhibiting stress-induced opening of the adherens junctions.28 The reason for such a discrepancy is not clear; therefore, the effect of β15-42–containing fragments on the endothelial barrier function requires further investigation. Whatever the reason is, our observation of the redistribution of VE-cadherin in HUVECs prompted us to hypothesize the involvement of a member of a RAP-dependent LDLR family in this process. Testing this hypothesis resulted in the discovery of a novel high-affinity interaction between fibrin and the VLDLR that occurs through fibrin βN-domains.
Our other findings, that RAP abrogates the effect of (β15-66)2 on the structure of HUVECs and inhibits NDSK-II–induced transendothelial migration of leukocytes, prompted us to hypothesize the involvement of fibrin-VLDLR interaction in fibrin-dependent leukocyte transmigration. Our recent study that used the in vivo peritonitis model showed that the dimeric (β15-66)2 fragment and its truncated variant (β15-44)2 both inhibited infiltration of leukocytes (neutrophils) from the circulation into the peritoneum, further confirming the role of fibrin or its degradation products in this process.15 In the present study, we used this model to test the aforementioned hypothesis by studying the effect of these fragments on the infiltration of neutrophils in wild-type and VLDLR−/− mice. Our observation that both fragments significantly inhibited neutrophil infiltration in wild-type mice, although having no effect in VLDLR−/− mice directly, confirms the important role of the VLDLR in supporting fibrin-induced leukocyte transmigration.
Our experiments indicate that the VLDLR interacts with immobilized fibrin through its βN-domains. Because such fibrin may be monomeric on the surface and may not mimic fibrin polymer, we also tested the interaction of VLDLR with fibrin-derived D-D:E1 complex, which preserves the structure and functional properties of the D and E regions of polymeric fibrin22 and is often used as a soluble model of fibrin polymers. This complex exhibited prominent binding to immobilized VLDLR, suggesting that the VLDLR-binding sites in polymeric fibrin are exposed. One can object that the D-D:E1 complex may mimic only fibrin protofibrils and that in fibrin fibers the N-terminal portions of the βN-domains, including the βGly15-Pro16-Arg17 sequence, are involved in the B:b interactions and, therefore, inaccessible. However, a previous study with the mAb T2G1 recognizing the β15-21 epitope reported that ≥ 14% of the available T2G1-reactive epitopes are exposed in fibrin clots.29 Furthermore, the VLDLR-binding site may be located in a different part of the βN-domain. Thus, the VLDLR should interact with both fibrin degradation products containing the βN-domains and fibrin polymers. Similarly, VE-cadherin, whose fibrin-binding site was localized in the N-terminal portion of the βN-domain,13 should also interact with fibrin clots, although this remains to be shown.
According to the current view, fibrin degradation products containing the β15-42 sequence promote leukocyte transmigration and thereby inflammation by bridging leukocytes to the endothelium through the interaction with the leukocyte receptor CD11c and endothelial cell VE-cadherin, and the anti-inflammatory effect of the β15-42 peptide is connected with its ability to inhibit the interaction with VE-cadherin.4,11,30 Taking into account the exposure of the VE-cadherin–binding sites in fibrin clots mentioned in the preceding paragraph one can speculate that fibrin deposited on the endothelium may also promote leukocyte transmigration. Therefore, this process may depend not only on fibrin degradation products, as was suggested earlier,4,11,30 but also on fibrin; that is, the latter may promote leukocyte transmigration by the same bridging mechanism. In the present study, we discovered that the VLDLR also modulates fibrin-dependent leukocyte transmigration. Whether fibrin-VLDLR interaction contributes to bridging of leukocytes to the endothelium and acts together with fibrin-VE-cadherin interaction or this interaction is a part of another fibrin-dependent mechanism of leukocyte transmigration remains to be established.
In summary, the present study showed that fibrin interacts with high affinity with the endothelial cell receptor VLDLR. The interaction occurs through fibrin βN-domains, is inhibited by RAP and by the dimeric fragments mimicking these domains, and is involved in fibrin-dependent transendothelial migration of leukocytes. Thus, we identified VLDLR as a novel endothelial cell receptor for fibrin that modulates fibrin-dependent leukocyte transmigration and thereby inflammation. Establishment of the molecular mechanism underlying this interaction and localization of VLDLR-binding site in fibrin βN-domain may result in the development of novel inhibitors of fibrin-dependent inflammation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health (grant HL-056051; L.M.), (grants HL-054710 and HL-050784; D.K.S.), and (grants HL-054710 and AI-078365; L.Z.).
National Institutes of Health
Authorship
Contribution: S.Y. prepared all protein fragments, performed all binding and transmigration studies, participated in immunofluorescence microscopy and animal studies, and contributed to experimental design, data analysis, and writing the manuscript; I.M. performed immunofluorescence microscopy study and contributed to writing the manuscript; C.C. performed animal experiments; L.Z. codesigned and coordinated animal experiments and contributed to data analysis and writing the manuscript; D.K.S. provided critical reagents and VLDLR-deficient mice and contributed to data analysis and writing the manuscript; and L.M. designed and coordinated all experiments, contributed to data analysis, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Leonid Medved, University of Maryland School of Medicine, Center for Vascular and Inflammatory Diseases, 800 W Baltimore St, Baltimore, MD 21201; e-mail: lmedved@som.umaryland.edu.



![Figure 4. Inhibitory effect of RAP on the interaction of the (β15-66)2 fragment with the VLDLR detected by SPR. (A) RAP (50nM; solid line), 500nM (β15-66)2 (dotted line), or their mixture [50nM RAP + 500nM (β15-66)2] (broken line), were added to immobilized VLDLR and their association/dissociation was monitored in real time. (B) The same mixture of RAP and (β15-66)2 was added after the addition of 50nM RAP (solid line) or 500nM (β15-66)2, (broken line); arrows indicate injection of the mixture. The curves are representative of 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/2/10.1182_blood-2011-09-382580/5/m_zh89991184400004.jpeg?Expires=1763459742&Signature=JtzqBdIFIJpWjxwhblWo8Zxi4t8eO7I1ytQRm5gqBeygtEtMEEytpfBr-cJHUCK1dBsxOkx7aCA5Lu3CzMQm0tHVSsgO2J3-aHSXf6KN9vj8K6M5HgblLYiHnWYu8nJWmj-LTIG9H~1HijQPmH66j0DmNT651c1h71fYMcfNfKzCN4UM6WwZbzcnH9a6m70xJEd1RLkSjapEoYq3hjDCw4vUqw548zOtVkGBWBfqPyH4cCNLK7pR0fcej8HkdvXQekEaXB13Aqm1DXIq1x0rWtgKzgT2Xudco447b4u0~HR3uEdrosuVPCDD4fYFmc7OCWIFj8zoGlrQdCtpvkOjiw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal