Abstract
Analysis of the chronic lymphocytic leukemia (CLL) coding genome has recently disclosed that the NOTCH1 proto-oncogene is recurrently mutated at CLL presentation. Here, we assessed the prognostic role of NOTCH1 mutations in CLL. Two series of newly diagnosed CLL were used as training (n = 309) and validation (n = 230) cohorts. NOTCH1 mutations occurred in 11.0% and 11.3% CLL of the training and validation series, respectively. In the training series, NOTCH1 mutations led to a 3.77-fold increase in the hazard of death and to shorter overall survival (OS; P < .001). Multivariate analysis selected NOTCH1 mutations as an independent predictor of OS after controlling for confounding clinical and biologic variables. The independent prognostic value of NOTCH1 mutations was externally confirmed in the validation series. The poor prognosis conferred by NOTCH1 mutations was attributable, at least in part, to shorter treatment-free survival and higher risk of Richter transformation. Although NOTCH1 mutated patients were devoid of TP53 disruption in more than 90% cases in both training and validation series, the OS predicted by NOTCH1 mutations was similar to that of TP53 mutated/deleted CLL. NOTCH1 mutations are an independent predictor of CLL OS, tend to be mutually exclusive with TP53 abnormalities, and identify cases with a dismal prognosis.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common leukemia in adults.1–4 The clinical course of CLL ranges from very indolent, with a nearly normal life expectancy,5–9 to rapidly progressive leading to death and occasionally undergoing transformation to aggressive lymphoma, known as Richter syndrome (RS).10–18
At presentation, several clinical and biologic features may help to predict, at least in part, the clinical course of CLL.19–21 Of the biologic prognosticators that have been developed, current guidelines for clinical practice recommend screening only for TP53 disruption by mutation, deletion, or both of the locus, that identifies a fraction of high-risk CLL destined to experience a very short survival.2,21–28 High-risk CLL, however, cannot be fully recapitulated by TP53 disruption, and other lesions of cancer genes may be implicated in this aggressive phenotype.29
Recently, two independent investigations of the CLL coding genome have revealed that activating mutations of the NOTCH1 proto-oncogene are recurrently associated with CLL.30,31 Based on current knowledge, NOTCH1 mutations occur in ∼ 10% CLL at diagnosis and their frequency increases in advanced disease phases, as exemplified by the case of RS.30,31 The relevance of NOTCH1 mutations in CLL is reinforced by knowledge of activation of the NOTCH1 pathway in this leukemia,32 and by the possibility of targeting NOTCH1 with drugs currently under development in other clinical contexts.33 Although not designed to fully assess clinical correlates, the pivotal studies that have identified NOTCH1 mutations in CLL have provided initial evidence suggesting that NOTCH1 alterations might be associated with an unfavorable clinical outcome.30,31,34 However, several aspects of the clinical implications of NOTCH1 mutations in CLL still remain to be elucidated, including: (1) their distribution among well established CLL genetic subgroups, including those defined by FISH abnormalities and TP53 status; and (2) their independent prognostic role, given the tight association between NOTCH1 mutations and unmutated immunoglobulin heavy variable (IGHV) genes, one of the most widely accepted prognosticators in CLL.
By using a training-validation approach, we hereby report that NOTCH1 mutations: (1) cluster with CLL harboring trisomy 12, suggesting that aberrant NOTCH signaling plays an important role in this genetic subgroup; (2) tend to be mutually exclusive with TP53 disruption in the same patient; and (3) are an independent predictor of CLL overall survival (OS) because they identify a subset of high-risk patients with dismal prognosis similar to that associated with TP53 abnormalities.
Patients and methods
Patients
The study used a training-validation design. The training cohort was a consecutive series of 309 previously untreated CLL who presented for initial evaluation at a single center. The training series was provided with prospectively collected biologic samples drawn at presentation and with a prospectively maintained clinical database updated in May 2010. Median follow-up of alive patients was 6 years. No patient was lost at follow-up. The validation cohort was represented by a retrospective series of 230 previously untreated CLL from 3 institutions participating to the same national CLL network. Inclusion criteria for the validation series were availability of: (1) biologic samples collected at presentation, and (2) clinical follow-up. Median follow-up of alive patients for the validation series was 7 years.
For sample size definition, we assumed a prevalence of NOTCH1 mutations at presentation of at least 10% and a 5-year OS of 80% for the entire population. Based on these assumptions, the sample size would allow detection of at least 15% and 19% difference in 5-year OS for the training series and the validation series, respectively (power = 80%; α = .05).
CLL diagnosis was based on International Working Group on CLL–National Cancer Institute criteria.1,2 RS diagnosis was histologically proven and was represented by diffuse large B-cell lymphoma (clonally related to the CLL phase).1,35
The Reporting Recommendations for Tumor Marker Prognostic Studies criteria were followed throughout this study.36 Patients provided informed consent in accordance with local institutional review board requirements and Declaration of Helsinki. The study was approved by the Ethical Committee of the Ospedale Maggiore della Carità di Novara associated with the Amedeo Avogadro University of Eastern Piedmont (protocol code 59/CE; study CE 8/11).
Molecular studies
NOTCH1, TP53, and IGHV mutations were analyzed by DNA Sanger sequencing.17,26,31,37,38 NOTCH1 c.7544_7545delCT mutation also was investigated by amplification refractory mutation system (ARMS) PCR. Probes (Abbott) used for FISH analysis were LSI13 and LSID13S319, CEP12, LSIp53, and LSIATM.18,23 Molecular studies were performed on tumor samples collected from peripheral blood: (1) at CLL presentation for both the training (n = 309) and validation (n = 230) CLL series; and (2) at the time of first progression requiring treatment for progressive CLL that were treated with fludarabine-based regimens (n = 113). Details of molecular methods are in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Flow cytometry
CD38 and ZAP-70 expression was analyzed by flow cytometry on PBMCs collected at presentation. Cut-off points of 30% and 20% were used to define positivity for CD38 and ZAP-70, respectively. Details are reported in supplemental Methods.
Serum β2-microglobulin quantification
β2-microglobulin levels were quantified by nephelometry (Siemens Healthcare Diagnostics; reference range, 1.8-2.5 mg/L) on serum samples collected at presentation.
Statistical analysis
OS was measured from date of initial presentation to date of death (event) or last follow-up (censoring). Treatment-free survival (TFS) was measured from date of initial presentation to date of progressive and symptomatic disease requiring treatment according to International Working Group on CLL–National Cancer Institute guidelines (event), death, or last follow-up (censoring).2 Time to RS transformation was measured from date of initial presentation to date of the biopsy documenting occurrence of RS transformation (event), death, or last follow-up (censoring).17 OS from first line treatment was assessed among cases treated with fludarabine-based regimens (n = 113) and was measured from date of treatment start to date of death (event) or last follow-up (censoring). Survival was estimated by Kaplan–Meier method.39 The crude association between exposure variables and outcome was estimated by univariate Cox regression analysis.40 The independence of NOTCH1 mutations as a predictor of CLL OS was estimated after controlling for confounding variables by multivariate Cox regression analysis.40–42
Covariates included in the multivariate analysis along with NOTCH1 mutation status were selected according to the following criteria: (1) wide acceptance as clinical or biologic prognosticators in CLL, (2) availability of the information in both the training and validation series, and (3) limitation of the number of predictors to no more than ∼ 1/10 uncensored events to avoid overfitting.41,42 Based on these criteria, the following variables were included in multivariate analysis: NOTCH1 mutations (present vs absent), age (continuous variable), sex (male vs female), Rai stage (III-IV vs 0-II), IGHV identity ≥ 98% (present vs absent), trisomy 12 (present vs absent), 11q22-q23 deletion (present vs absent), and TP53 disruption by mutation, deletion, or both (present vs absent). None of the covariates violated the proportional hazard assumption as documented by plotting the smoothed Schoenfeld residuals and by performing a correlation test between time and residuals.41–43 The assumption of effect additivity of predictors was not violated, as documented by a global test of additivity including interactions between NOTCH1 mutations and other covariates.41,42 None of the covariates showed colinearity.41,42 Age was treated as a continuous variable and did not violate the linearity assumption as assessed by plotting the smoothed martingale residuals.41,42,44
The prediction accuracy of the multivariate model was verified by assessing model discrimination and calibration (see supplemental Methods for details).41,42,45 The stability and predictive performance of NOTCH1 mutations as an independent predictor of CLL OS was validated both internally in the training series and externally in an independent validation series. Internal validation was performed using a bootstrapping resampling procedure (see supplemental Methods for details).41,42,46 The more general validity of NOTCH1 mutations as an independent predictor of CLL OS was tested using an external validation approach. In this step, Cox regression was applied to an independent validation cohort that included NOTCH1 mutations and the confounding variables also tested in the training series.
Recursive-partitioning analysis for censored survival data was performed to hierarchically classify CLL patients into risk categories based on NOTCH1 and TP53 status.47 Categorical variables were compared by χ2 test and Fisher exact test when appropriate. Continuous variables were compared by Mann-Whitney test. All statistical tests were 2-sided. Statistical significance was defined as P value < .05. The analysis was performed with the Statistical Package for the Social Sciences Version 18.0 software (SPSS) and with R statistical package 2.13.0 (http://www.r-project.org).
Results
Frequency and distribution of NOTCH1 mutations in the training series
The training series (n = 309) was representative of the main clinical and biologic characteristics of CLL (Table 1). NOTCH1 mutations (n = 34, all heterozygous) occurred in 34/309 (11.0%) patients, being mostly represented (26/34, 76.5%) by a recurrent 2-bp frameshift deletion (c.7544_7545delCT). The remaining NOTCH1 mutations (8/34, 23.5%) were frameshift deletions other than c.7544_7545delCT (n = 7) and frameshift insertions (n = 1; supplemental Table 1). All mutations were predicted to disrupt the NOTCH1 PEST domain.
Characteristics of the CLL training series according to NOTCH1 mutation status
| Characteristic . | All (n = 309) . | NOTCH1 wild type (n = 275) . | NOTCH1 mutated (n = 34) . | P . | |||
|---|---|---|---|---|---|---|---|
| n* . | % . | n* . | % . | n* . | % . | ||
| Age, y (range) | 69 (60-76) | 69 (60-76) | 69 (60-76) | .615 | |||
| Male | 170 | 55.0 | 152 | 55.3 | 18 | 52.9 | .797 |
| Rai stage III-IV | 34 | 11.0 | 25 | 9.1 | 9 | 26.5 | .006 |
| IGHV identity ≥ 98% | 103 | 33.3 | 77 | 28.0 | 26 | 76.5 | < .001 |
| 13q14 deletion | 160 | 51.8 | 151 | 54.9 | 9 | 26.5 | .002 |
| Normal FISH | 92 | 29.8 | 81 | 29.5 | 11 | 32.4 | .727 |
| Trisomy 12 | 61 | 19.7 | 46 | 16.7 | 15 | 44.1 | < .001 |
| 11q22-q23 deletion | 25 | 8.1 | 24 | 8.7 | 1 | 2.9 | .334 |
| 17p13 deletion | 25 | 8.1 | 24 | 8.7 | 1 | 2.9 | .334 |
| TP53 mutations | 23 | 7.4 | 21 | 7.6 | 2 | 5.9 | 1.000 |
| TP53 disruption | 33 | 10.7 | 30 | 10.9 | 3 | 8.8 | 1.000 |
| Characteristic . | All (n = 309) . | NOTCH1 wild type (n = 275) . | NOTCH1 mutated (n = 34) . | P . | |||
|---|---|---|---|---|---|---|---|
| n* . | % . | n* . | % . | n* . | % . | ||
| Age, y (range) | 69 (60-76) | 69 (60-76) | 69 (60-76) | .615 | |||
| Male | 170 | 55.0 | 152 | 55.3 | 18 | 52.9 | .797 |
| Rai stage III-IV | 34 | 11.0 | 25 | 9.1 | 9 | 26.5 | .006 |
| IGHV identity ≥ 98% | 103 | 33.3 | 77 | 28.0 | 26 | 76.5 | < .001 |
| 13q14 deletion | 160 | 51.8 | 151 | 54.9 | 9 | 26.5 | .002 |
| Normal FISH | 92 | 29.8 | 81 | 29.5 | 11 | 32.4 | .727 |
| Trisomy 12 | 61 | 19.7 | 46 | 16.7 | 15 | 44.1 | < .001 |
| 11q22-q23 deletion | 25 | 8.1 | 24 | 8.7 | 1 | 2.9 | .334 |
| 17p13 deletion | 25 | 8.1 | 24 | 8.7 | 1 | 2.9 | .334 |
| TP53 mutations | 23 | 7.4 | 21 | 7.6 | 2 | 5.9 | 1.000 |
| TP53 disruption | 33 | 10.7 | 30 | 10.9 | 3 | 8.8 | 1.000 |
Median and 25th-75th percentiles are reported for continuous variables.
The clinical and biologic features of NOTCH1 mutated CLL are summarized in Table 1. CLL with NOTCH1 mutations preferentially carried unmutated IGHV genes (76.5%; P < .001). Other characteristics at presentation associated with NOTCH1 mutations were advanced Rai stage and trisomy 12 (Table 1). Consistent with the mutually exclusive distribution of trisomy 12 and 13q14 deletion,23 NOTCH1 mutated CLL were less frequently deleted on 13q14 (Table 1).
NOTCH1 mutations are an independent prognosticator of OS in the training series
After a median follow-up of 6 years, 135/309 patients from the training series had received treatment, 19/309 had developed RS and 78/309 had died, accounting for a median TFS of 7.1 year (95% confidence interval [CI], 4.5-9.8), a 5-year risk of RS of 7.9% (95% CI, 4.4-11.4), and a median OS of 13.0 years (95% CI, 10.2-15.9).
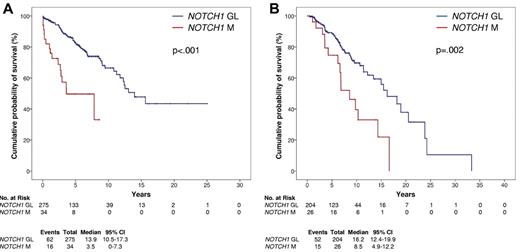
By univariate analysis, the crude impact of NOTCH1 mutations on survival was an ∼ 3.8-fold increase in the hazard ratio (HR, 3.77; 95% CI, 2.14-6.66) and a significant OS shortening (P < .001; Table 2; Figure 1A) that occurred irrespective of the NOTCH1 mutation type (c.7544_7545delCT, P < .001; other mutations, P = .009; supplemental Figure 1). Other variables associated with shorter OS were age, Rai stage, IGHV mutation status, trisomy 12, 11q22-q23 deletion, and TP53 disruption (Table 2; supplemental Figure 2).
Univariate and multivariate analysis for overall survival in the CLL training series
| Characteristics . | Event . | Total . | OS, y . | Univariate analysis . | Multivariate analysis*†‡ . | Internal bootstrapping validation . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bootstrap parameter, mean . | Bootstrap selection, % . | ||||||||||||||||
| Median . | LCI . | UCI . | HR . | LCI . | UCI . | P . | HR . | LCI . | UCI . | P . | HR . | LCI . | UCI . | ||||
| Age§ | 1.06 | 1.03 | 1.08 | < .001 | 1.07 | 1.04 | 1.10 | < .001 | 1.07 | 1.05 | 1.10 | 100 | |||||
| Female | 29 | 139 | 13.9 | 10.8 | 17.0 | ||||||||||||
| Male | 49 | 170 | 12.2 | 8.0 | 16.5 | 1.53 | .096 | 2.43 | .069 | 1.96 | 1.20 | 3.20 | .007 | 2.17 | 1.28 | 3.68 | 91.1 |
| NOTCH1 germline | 62 | 275 | 13.9 | 10.5 | 17.3 | ||||||||||||
| NOTCH1 mutations | 16 | 34 | 3.5 | 0 | 7.3 | 3.77 | 2.14 | 6.66 | < .001 | 3.99 | 2.05 | 7.76 | < .001 | 4.55 | 2.23 | 9.31 | 98.0 |
| Rai stage 0-II | 55 | 275 | 15.6 | 13.1 | 18.7 | ||||||||||||
| Rai stage III-IV | 23 | 34 | 6.0 | 2.5 | 9.6 | 3.99 | 2.44 | 6.50 | < .001 | 2.33 | 1.25 | 3.99 | .007 | 2.57 | 1.38 | 4.82 | 90.0 |
| IGHV identity < 98% | 42 | 206 | NR | ||||||||||||||
| IGHV identity ≥ 98% | 36 | 103 | 11.7 | 6.3 | 17.2 | 2.23 | 1.42 | 3.52 | .001 | 1.44 | 0.83 | 2.50 | .191 | 1.53 | 0.86 | 2.71 | 50.4 |
| No trisomy 12 | 52 | 248 | NR | ||||||||||||||
| Trisomy 12 | 26 | 61 | 10.8 | 5.5 | 16.0 | 1.93 | 1.21 | 3.01 | .006 | 1.50 | 0.91 | 2.64 | .108 | 1.62 | 0.96 | 2.74 | 55.4 |
| No 11q22-q23 deletion | 65 | 284 | 15.6 | 13.3 | 17.9 | ||||||||||||
| 11q22-q23 deletion | 13 | 25 | 6.8 | 0 | 14.1 | 2.25 | 1.23 | 4.11 | .008 | 1.57 | 0.76 | 3.27 | .220 | 1.77 | 0.81 | 3.90 | 47.4 |
| TP53 germline | 61 | 276 | 13.9 | 10.5 | 17.3 | ||||||||||||
| TP53 disruption | 17 | 33 | 4.6 | 1.8 | 7.3 | 3.74 | 2.16 | 6.49 | < .001 | 3.27 | 1.80 | 5.95 | < .001 | 3.38 | 1.78 | 6.43 | 96.5 |
| Characteristics . | Event . | Total . | OS, y . | Univariate analysis . | Multivariate analysis*†‡ . | Internal bootstrapping validation . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bootstrap parameter, mean . | Bootstrap selection, % . | ||||||||||||||||
| Median . | LCI . | UCI . | HR . | LCI . | UCI . | P . | HR . | LCI . | UCI . | P . | HR . | LCI . | UCI . | ||||
| Age§ | 1.06 | 1.03 | 1.08 | < .001 | 1.07 | 1.04 | 1.10 | < .001 | 1.07 | 1.05 | 1.10 | 100 | |||||
| Female | 29 | 139 | 13.9 | 10.8 | 17.0 | ||||||||||||
| Male | 49 | 170 | 12.2 | 8.0 | 16.5 | 1.53 | .096 | 2.43 | .069 | 1.96 | 1.20 | 3.20 | .007 | 2.17 | 1.28 | 3.68 | 91.1 |
| NOTCH1 germline | 62 | 275 | 13.9 | 10.5 | 17.3 | ||||||||||||
| NOTCH1 mutations | 16 | 34 | 3.5 | 0 | 7.3 | 3.77 | 2.14 | 6.66 | < .001 | 3.99 | 2.05 | 7.76 | < .001 | 4.55 | 2.23 | 9.31 | 98.0 |
| Rai stage 0-II | 55 | 275 | 15.6 | 13.1 | 18.7 | ||||||||||||
| Rai stage III-IV | 23 | 34 | 6.0 | 2.5 | 9.6 | 3.99 | 2.44 | 6.50 | < .001 | 2.33 | 1.25 | 3.99 | .007 | 2.57 | 1.38 | 4.82 | 90.0 |
| IGHV identity < 98% | 42 | 206 | NR | ||||||||||||||
| IGHV identity ≥ 98% | 36 | 103 | 11.7 | 6.3 | 17.2 | 2.23 | 1.42 | 3.52 | .001 | 1.44 | 0.83 | 2.50 | .191 | 1.53 | 0.86 | 2.71 | 50.4 |
| No trisomy 12 | 52 | 248 | NR | ||||||||||||||
| Trisomy 12 | 26 | 61 | 10.8 | 5.5 | 16.0 | 1.93 | 1.21 | 3.01 | .006 | 1.50 | 0.91 | 2.64 | .108 | 1.62 | 0.96 | 2.74 | 55.4 |
| No 11q22-q23 deletion | 65 | 284 | 15.6 | 13.3 | 17.9 | ||||||||||||
| 11q22-q23 deletion | 13 | 25 | 6.8 | 0 | 14.1 | 2.25 | 1.23 | 4.11 | .008 | 1.57 | 0.76 | 3.27 | .220 | 1.77 | 0.81 | 3.90 | 47.4 |
| TP53 germline | 61 | 276 | 13.9 | 10.5 | 17.3 | ||||||||||||
| TP53 disruption | 17 | 33 | 4.6 | 1.8 | 7.3 | 3.74 | 2.16 | 6.49 | < .001 | 3.27 | 1.80 | 5.95 | < .001 | 3.38 | 1.78 | 6.43 | 96.5 |
LCI indicates 95% lower confidence interval; UCI, 95% upper confidence interval; and NR, not reached.
Shrinkage coefficient, 0.91.
Discrimination: c-index of the original model, 0.792; bias-corrected c-index, 0.015; and optimism, 0.777.
Calibration: calibration slope of the original model, 1.000; bias-corrected calibration slope, 0.910; and optimism, 0.090.
Age was treated as a continuous variable.
Kaplan-Meier estimates of OS according to NOTCH1 mutation status. Overall survival according to NOTCH1 mutation status in the CLL training series (n = 309; A) and in the CLL validation series (n = 230; B). NOTCH1 germ line cases (NOTCH1 GL) are represented by the blue line. NOTCH1 mutated cases (NOTCH1 M) are represented by the red line.
Kaplan-Meier estimates of OS according to NOTCH1 mutation status. Overall survival according to NOTCH1 mutation status in the CLL training series (n = 309; A) and in the CLL validation series (n = 230; B). NOTCH1 germ line cases (NOTCH1 GL) are represented by the blue line. NOTCH1 mutated cases (NOTCH1 M) are represented by the red line.
The adjusted impact of NOTCH1 mutations on OS was estimated after controlling for confounding variables by multivariate Cox regression analysis. Along with NOTCH1 mutations, other variables included in the analysis were: (1) those known a priori to be widely accepted clinical (age and Rai stage)3–14 and genetic (IGHV mutation status, 11q22-q23 deletion, and TP53 disruption)20–28,37,38 risk factors affecting CLL OS; (2) trisomy 12, given its double association with NOTCH1 mutations and OS in this series (Tables 1–2); and (3) sex.
Multivariate analysis selected NOTCH1 mutations as an independent risk factor of OS (HR, 4.22; 95% CI, 2.15-8.28; P < .001; Table 2). The inclusion of NOTCH1 mutations in addition to age, sex, Rai stage, IGHV mutation status, trisomy 12, 11q22-q23 deletion, and TP53 disruption significantly improved the fit (-2LL of the model without NOTCH1 mutations, 692 vs -2LL of the model with NOTCH1 mutations, 677; likelihood ratio statistics, 14.5; P < .001) and the predictive accuracy (c-index of the model without NOTCH1 mutations, 0.768 vs c-index of the model with NOTCH1 mutations, 0.792; P < .001) of the model. The IGHV mutation status that was significant in the model without NOTCH1 mutations (supplemental Table 2) was no longer retained as an independent prognosticator of OS after inclusion of NOTCH1 mutations. By bivariate analysis, the IGHV mutation status maintained its prognostic relevance in CLL devoid of NOTCH1 mutations (supplemental Figure 3).
The stability and predictive performance of NOTCH1 mutations as an independent prognostic factor of CLL OS was internally validated in the training series using a bootstrapping resampling procedure. The first step of the internal validation showed that NOTCH1 mutations were selected at high frequency (> 98.3%) as an independent prognosticator of CLL OS in each of the 1000 bootstrap samples that were generated (Table 2). This step validated NOTCH1 mutations as one of the most important variables affecting OS in the training series. The second step of the internal validation demonstrated that the hazard ratios produced from the original series were very close to those produced from the 1000 bootstrap samples (Table 2).
An exploratory analysis applied only to the training series demonstrated that NOTCH1 mutations maintained their independent prognostic role also after adjusting for β2-microglobulin levels, ZAP-70 expression, and CD38 expression (supplemental Table 3).
NOTCH1 mutations predict an increased risk of CLL progression, RS transformation, and short survival after treatment
At CLL diagnosis, NOTCH1 mutations identified CLL patients with rapidly progressive disease and patients at risk of RS development. In fact, patients from the training series carrying NOTCH1 mutations displayed a shorter time to progression requiring treatment compared with patients without NOTCH1 mutations (P < .001; Figure 2A). In addition, NOTCH1 mutated patients from the training series displayed a higher cumulative probability of RS compared with patients without NOTCH1 mutations (P = .026; Figure 2B), that occurred irrespective of the NOTCH1 mutation type.
Kaplan-Meier estimates of TFS and cumulative probability of transformation to RS according to NOTCH1 mutation status. TFS according to NOTCH1 mutation status (A) and cumulative probability of transformation to RS (B) in the CLL training series (n = 309). NOTCH1 germ line cases (NOTCH1 GL) are represented by the blue line. NOTCH1 mutated cases (NOTCH1 M) are represented by the red line.
Kaplan-Meier estimates of TFS and cumulative probability of transformation to RS according to NOTCH1 mutation status. TFS according to NOTCH1 mutation status (A) and cumulative probability of transformation to RS (B) in the CLL training series (n = 309). NOTCH1 germ line cases (NOTCH1 GL) are represented by the blue line. NOTCH1 mutated cases (NOTCH1 M) are represented by the red line.
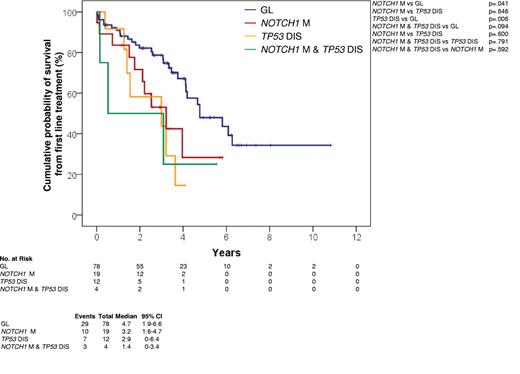
In CLL patients treated with fludarabine-based regimens at first progression requiring treatment, NOTCH1 mutations occurred in 23/113 (20.4%) cases (supplemental Table 4) and associated with an OS from treatment similar to that marked by TP53 disruption (P = .600) and significantly shorter compared with that of patients lacking both NOTCH1 and TP53 lesions (P = .041; Figure 3).
Kaplan-Meier estimates of OS from first-line treatment. OS from first line treatment according to NOTCH1 mutation status in CLL treated with fludarabine-based regimens series (n = 113). Cases with germ line NOTCH1 and TP53 genes (GL) are represented by the blue curve. Cases harboring NOTCH1 mutations without TP53 disruption (NOTCH1 M) are represented by the red curve. Cases harboring TP53 disruption without NOTCH1 mutations (TP53 DIS) are represented by the yellow curve. Cases harboring both NOTCH1 mutations and TP53 disruption (NOTCH1 M and TP53 DIS) are represented by the green curve.
Kaplan-Meier estimates of OS from first-line treatment. OS from first line treatment according to NOTCH1 mutation status in CLL treated with fludarabine-based regimens series (n = 113). Cases with germ line NOTCH1 and TP53 genes (GL) are represented by the blue curve. Cases harboring NOTCH1 mutations without TP53 disruption (NOTCH1 M) are represented by the red curve. Cases harboring TP53 disruption without NOTCH1 mutations (TP53 DIS) are represented by the yellow curve. Cases harboring both NOTCH1 mutations and TP53 disruption (NOTCH1 M and TP53 DIS) are represented by the green curve.
NOTCH1 mutations are an independent prognosticator of OS in the validation series
The prognostic value of NOTCH1 mutations as a risk factor was externally validated in an independent CLL series (n = 230; supplemental Table 5). NOTCH1 mutations (n = 26, all heterozygous) occurred in 26/230 (11.3%) patients and affected in all cases the PEST domain, with a mutational spectrum similar to that of the training series (c.7544_7545delCT, 21/26 [80.7%]; other mutations, 5/26 [19.3%]; supplemental Table 1). Survival analysis in the validation series confirmed that NOTCH1 mutations represent an adverse prognostic factor in CLL. By univariate analysis, NOTCH1 mutated patients were confirmed to display a significantly shorter OS compared with NOTCH1 germ line patients (P = .002; Figure 1B). Similarly to the training series, also in the validation series NOTCH1 mutations predicted poor OS irrespective of mutation type (supplemental Figure 1). By multivariate analysis, NOTCH1 mutations were selected as an independent risk factor of OS also in the validation series (HR, 2.15; 95% CI, 1.13-4.11; P = .019), after controlling for the same covariates applied to the training series.
CLL with NOTCH1 mutations have a poor prognosis similar to CLL with TP53 disruption
NOTCH1 mutations and TP53 disruption tended to distribute in a mutually exclusive fashion in both the training and validation series (Figure 4A-B). Patients harboring NOTCH1 mutations were devoid of TP53 disruption in 31/34 (91.2%) and in 25/26 (96.2%) cases of the training and validation series, respectively.
Comparative analysis of NOTCH1 mutations and TP53 disruption. (A-B) Venn diagram illustrating the overlap between NOTCH1 mutations and TP53 disruption by mutations, deletion, or both in the CLL training series (A) and in the CLL validation series (B). Numbers within the red and yellow circles indicate the number of cases harboring NOTCH1 mutations without TP53 disruption (red circle) and cases with TP53 disruption without NOTCH1 mutations (yellow circle). Numbers within the overlaps between circles indicate the number of cases harboring both NOTCH1 mutations and TP53 disruption. (C-D) Kaplan–Meier estimates of OS according to NOTCH1 mutation and TP53 disruption in the CLL training series (n = 309; C) and in the CLL validation series (n = 230; D). Cases with germ line NOTCH1 and TP53 genes (GL) are represented by the blue line. Cases harboring NOTCH1 mutations without TP53 disruption (NOTCH1 M) are represented by the red line. Cases harboring TP53 disruption (TP53 DIS) are represented by the yellow line. Cases harboring both NOTCH1 and TP53 disruption were compiled to cases harboring only TP53 disruption, because of the low number (3 in the training series and 1 in the validation series) of double-mutated cases, and based on a recursive partitioning analysis for risk of death.
Comparative analysis of NOTCH1 mutations and TP53 disruption. (A-B) Venn diagram illustrating the overlap between NOTCH1 mutations and TP53 disruption by mutations, deletion, or both in the CLL training series (A) and in the CLL validation series (B). Numbers within the red and yellow circles indicate the number of cases harboring NOTCH1 mutations without TP53 disruption (red circle) and cases with TP53 disruption without NOTCH1 mutations (yellow circle). Numbers within the overlaps between circles indicate the number of cases harboring both NOTCH1 mutations and TP53 disruption. (C-D) Kaplan–Meier estimates of OS according to NOTCH1 mutation and TP53 disruption in the CLL training series (n = 309; C) and in the CLL validation series (n = 230; D). Cases with germ line NOTCH1 and TP53 genes (GL) are represented by the blue line. Cases harboring NOTCH1 mutations without TP53 disruption (NOTCH1 M) are represented by the red line. Cases harboring TP53 disruption (TP53 DIS) are represented by the yellow line. Cases harboring both NOTCH1 and TP53 disruption were compiled to cases harboring only TP53 disruption, because of the low number (3 in the training series and 1 in the validation series) of double-mutated cases, and based on a recursive partitioning analysis for risk of death.
Because TP53 disruption identifies patients with the shortest survival in CLL,22–28 the outcome of NOTCH1 mutated cases was compared with that of patients with TP53 disruption. Cases harboring both NOTCH1 and TP53 lesions were compiled to cases harboring only TP53 disruption, because of the low number (3 in the training series and 1 in the validation series) of double mutated cases, and based on a recursive partitioning analysis for risk of death. In both the training and validation series, CLL harboring NOTCH1 mutations displayed an OS similar to that of CLL harboring TP53 disruption (Figure 4C-D). The results were superimposable also when the few double mutated cases were analyzed as a separate subgroup (supplemental Figure 4).
ARMS is a useful tool for NOTCH1 mutation screening
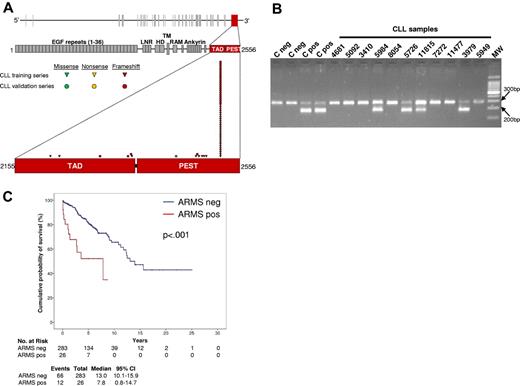
A PCR-based test was designed to detect the c.7544_7545delCT mutation that accounts for ∼ 80% of NOTCH1 mutations in CLL. ARMS was calibrated to detect a mutation present in great than or equal to 10% alleles, approximating the sensitivity of DNA Sanger sequencing, and was applied in blind to the training series. Under these conditions, ARMS showed a 100% sensitivity and specificity in detecting NOTCH1 c.7544_7545delCT (κ = 1). In fact, all 26 CLL from the training series harboring c.7544_7545delCT by DNA Sanger sequencing scored positive by ARMS, whereas all 283 CLL lacking c.7544_7545delCT by DNA Sanger sequencing scored negative by ARMS (Figure 5). By survival analysis, CLL that scored positive by ARMS for c.7544_7545delCT showed a shorter OS compared with negative patients (P < .001; Figure 5). These results confirm that ARMS is a useful tool for NOTCH1 c.7544_7545delCT mutation screening.
ARMS to detect NOTCH1 c.7544_7545delCT mutation. (A) Schematic diagram of the NOTCH1 gene (top) and protein (bottom), with its conserved functional domains (EGF-like repeats: LNR, LIN-12/NOTCH repeats; HD, heterodimerization; TM, transmembrane; Ankyrin repeats: TAD, transactivation domain; PEST, proline, glutamic acid, serine, threonine sequence). The TAD domain and the PEST sequence, both coded by exon 34, are magnified. Color-coded shapes indicate the position of the mutations found in the CLL training series (n = 34) and in the CLL validation series (n = 26). (B) Representative results of the ARMS assay showing 4 CLL samples that scored positive for the c.7544_7545delCT mutation (codes 5984, 5726, 11815, 3979) and 7 CLL samples that scored negative for the c.7544_7545delCT mutation (codes 4681, 5092, 3410, 8054, 7272, 11477, 5949). Negative samples show a normal band of 284 bp. Positive samples show an additional mutant band of 183 bp. Negative (C neg) and positive (C pos) controls also are included. Molecular weight (MW) is the 100-bp DNA ladder. Camera: Gel Doc 1000, BioRad; image acquisition software: Quantity One 4.5.0, BioRad. (C) Kaplan-Meier estimates of overall survival according to the results of the ARMS assay in the CLL training series (n = 309). Cases that scored negative by ARMS for the NOTCH1 c.7544_7545delCT mutation (ARMS neg) are represented by the blue line. Cases that scored positive by ARMS for the NOTCH1 c.7544_7545delCT mutation (ARMS pos) are represented by the red line.
ARMS to detect NOTCH1 c.7544_7545delCT mutation. (A) Schematic diagram of the NOTCH1 gene (top) and protein (bottom), with its conserved functional domains (EGF-like repeats: LNR, LIN-12/NOTCH repeats; HD, heterodimerization; TM, transmembrane; Ankyrin repeats: TAD, transactivation domain; PEST, proline, glutamic acid, serine, threonine sequence). The TAD domain and the PEST sequence, both coded by exon 34, are magnified. Color-coded shapes indicate the position of the mutations found in the CLL training series (n = 34) and in the CLL validation series (n = 26). (B) Representative results of the ARMS assay showing 4 CLL samples that scored positive for the c.7544_7545delCT mutation (codes 5984, 5726, 11815, 3979) and 7 CLL samples that scored negative for the c.7544_7545delCT mutation (codes 4681, 5092, 3410, 8054, 7272, 11477, 5949). Negative samples show a normal band of 284 bp. Positive samples show an additional mutant band of 183 bp. Negative (C neg) and positive (C pos) controls also are included. Molecular weight (MW) is the 100-bp DNA ladder. Camera: Gel Doc 1000, BioRad; image acquisition software: Quantity One 4.5.0, BioRad. (C) Kaplan-Meier estimates of overall survival according to the results of the ARMS assay in the CLL training series (n = 309). Cases that scored negative by ARMS for the NOTCH1 c.7544_7545delCT mutation (ARMS neg) are represented by the blue line. Cases that scored positive by ARMS for the NOTCH1 c.7544_7545delCT mutation (ARMS pos) are represented by the red line.
Timing of NOTCH1 mutations and relationship with TP53 disruption in high-risk CLL
Paired sequential samples were tested in selected cases of high-risk CLL. NOTCH1 mutations were acquired at chemorefractoriness in 1/4 cases and at RS transformation in 4/11 (supplemental Table 6).
The relationship between NOTCH1 mutations and TP53 disruption was investigated in high-risk CLL, including fludarabine-refractory CLL (n = 59) and RS (n = 26; Figure 6). At the time of fludarabine-refractoriness, NOTCH1 mutations and TP53 disruption overlapped in 11.8% (7/59) of cases (Figure 6B). Consistent with the whole CLL series, NOTCH1 mutations and TP53 disruption distributed in a mutually exclusive manner also in CLL that subsequently transformed to RS (Figure 6A). However, on RS transformation, NOTCH1 mutations and TP53 disruption occurred simultaneously in a fraction of patients as documented by: (1) acquisition of TP53 disruption in 2 RS that already harbored NOTCH1 mutations in the CLL phase; and (2) acquisition of both NOTCH1 mutations and TP53 disruption in 3 RS devoid of these alterations in the CLL phase (Figure 6A).
Timing of NOTCH1 mutations and relationship with TP53 disruption in high-risk CLL. (A) NOTCH1 mutations and TP53 disruption in fludarabine-refractory CLL. In the heatmap, rows correspond to the NOTCH1 and TP53 genes, and columns represent individual patients color-coded based on the gene status (white, wild type; red, mutations of NOTCH1 and disruption of TP53). (B) NOTCH1 mutations and TP53 disruption in sequential CLL/RS samples. In the heatmap, rows correspond to the NOTCH1 and TP53 genes. Columns represent individual patients color-coded based on the gene status (white, wild type; pink, mutations of NOTCH1 and disruption of TP53 in the CLL phase; red, mutations of NOTCH1 and disruption of TP53 at RS transformation).
Timing of NOTCH1 mutations and relationship with TP53 disruption in high-risk CLL. (A) NOTCH1 mutations and TP53 disruption in fludarabine-refractory CLL. In the heatmap, rows correspond to the NOTCH1 and TP53 genes, and columns represent individual patients color-coded based on the gene status (white, wild type; red, mutations of NOTCH1 and disruption of TP53). (B) NOTCH1 mutations and TP53 disruption in sequential CLL/RS samples. In the heatmap, rows correspond to the NOTCH1 and TP53 genes. Columns represent individual patients color-coded based on the gene status (white, wild type; pink, mutations of NOTCH1 and disruption of TP53 in the CLL phase; red, mutations of NOTCH1 and disruption of TP53 at RS transformation).
Discussion
The current study on 539 CLL documents that NOTCH1 mutations: (1) represent one of the most frequent cancer gene mutations known to be involved at CLL presentation; (2) among CLL genetic subgroups, cluster with cases harboring trisomy 12 and tend to be mutually exclusive with TP53 disruption; (3) identify a high-risk subgroup of patients showing poor survival similar to that associated with TP53 abnormalities; and (4) exert a prognostic role independent of widely accepted clinical and genetic risk factors, and in series from different institutions, as documented by the training-validation approach chosen for the design of this study.
Of the biologic predictors of CLL identified to date,19–21 TP53 disruption is the sole risk factor consistently associated with high-risk patients.22–29 The genetics of high-risk CLL, however, is not fully recapitulated by TP53 disruption, because 40% to 50% high-risk CLL are devoid of TP53 abnormalities.29 Conceivably, other genetic lesions may drive CLL aggressiveness. This study is consistent with a role of NOTCH1 mutations in contributing to CLL clinical aggressiveness, because these genetic alterations identify patients whose survival is similar to that associated with TP53 disruption.
The role of NOTCH1 mutations in determining CLL aggressiveness is independent of the effect exerted by TP53 disruption. In fact, at presentation, NOTCH1 mutations in both the training and validation series tend to distribute in a mutually exclusive manner with TP53 disruption. Consistently, the impact of NOTCH1 mutations on CLL survival is independent of TP53 disruption by multivariate analysis. The scenario observed in CLL differs from that of RS, in which mutations of NOTCH1 associate with TP53 disruption in 50% of the patients (Fabbri et al31; this study). A likely explanation is that the concomitant occurrence of NOTCH1 mutations and TP53 disruption in the same CLL clone causes further clinical aggressiveness and, potentially, histologic transformation to aggressive lymphoma.
The pivotal studies on NOTCH1 mutations in CLL have provided initial evidence that NOTCH1 alterations might be associated with an unfavorable clinical outcome.30,31,34 However, these studies were based on small CLL series,34 used TFS as surrogate clinical end point,34 and lacked a formal demonstration that the clinical effect of NOTCH1 mutations is reproducible and independent of confounders.30,31 Our results add to the current knowledge on the clinical aspects of NOTCH1 mutations in CLL by demonstrating the robustness and reproducibility of these genetic alterations as a risk factor, thus providing a new tool for the early identification of high-risk patients.
Different mechanisms might explain, at least in part, the poor prognosis associated with NOTCH1 mutations in CLL. First, NOTCH1 mutations lead to the acquisition of a progressive clinical phenotype that mandates treatment shortly after initial presentation, as documented by a median TFS of ∼ 2 years for mutated cases versus ∼ 9 years for NOTCH1 germ line patients. Second, our actuarial analysis indicates that NOTCH1 mutated patients display a higher risk of developing RS, a condition that is frequently lethal and recurrently harbors NOTCH1 mutations that, importantly, are present already at the time of CLL presentation in a significant fraction of RS patients.15–18,31 A potential association of NOTCH1 mutations with chemorefractoriness may further explain the poor outcome associated with NOTCH1 alterations. Although the relationship between NOTCH1 mutations and response to treatment needs to be formally tested within clinical trials, indirect evidence for this hypothesis comes from the observation that NOTCH1 mutations are enriched among chemorefractory CLL patients31 and that NOTCH1 activation in vitro confers resistance to apoptosis through NF-κB pathway activation.32
The external validation approach exploited in the current study documents that NOTCH1 mutations are an independent prognostic factor retaining its predictive value in CLL followed at different institutions. This observation suggests that the prognostic value of NOTCH1 mutations, though detected retrospectively, is independent of a potential bias because of patient referral or patient management at a single center.42 The general validity of NOTCH1 mutations as a prognosticator in CLL is further supported by the consistent association of NOTCH1 mutations with poor outcome in all series tested to date by independent investigators, although previous studies were not designed for a comprehensive survival analysis, or included a limited number of patients.30,31,34 Confirmation within the frame of prospective clinical trials will be helpful to fully assess the generalization of NOTCH1 mutations as a prognostic marker in CLL.
Consistent with the mutational spectrum of NOTCH1 in CLL, all mutations disrupted the C-terminal PEST domain that in normal conditions is required to limit the intensity and duration of NOTCH1 activation.33,48,49 Removal of the PEST domain results in NOTCH1 impaired degradation and accumulation of an active NOTCH1 isoform sustaining deregulated signaling.30 A practically important feature of the NOTCH1 mutational spectrum is that one single recurrent mutation, c.7544_7545delCT, accounts for ∼ 80% of all NOTCH1 mutations detectable in CLL. The high recurrence of c.7544_7545delCT in CLL has prompted the design of a simple PCR-based strategy for its rapid detection. This assay allows the reliable detection of all cases harboring the c.7544_7545delCT mutation, translates into prognostically meaningful results, and might provide a potentially helpful approach for a first-level screening of NOTCH1 alterations avoiding the need of DNA sequencing procedures. In addition to prognostic implications, NOTCH1 mutations might also provide a therapeutic target for NOTCH1 inhibitors that are currently under development in other clinical contexts33,49 and that prompt future studies of molecular therapy for NOTCH1-mutated CLL patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by Associazione Italiana Ricerca sul Cancro, Special Program Molecular Clinical Oncology, 5 × 1000, No. 10007, Milan, Italy (G.G. and R.F.); Progetto Fondo per gli Investimenti della Ricerca di Base-Programma “Futuro in Ricerca” 2008 (D.R.), PRIN 2008 (G.G. and R.M.), and PRIN 2009 (D.R.), Ministero dell'Istruzione, dell'Università e della Ricerca, Rome, Italy; Progetto Giovani Ricercatori 2008 (D.R.) and Ricerca Sanitaria Finalizzata 2008 (G.G.), Ministero della Salute, Rome, Italy; Novara-AIL Onlus, Novara, Italy (G.G.); Compagnia di San Paolo, Turin, Italy (R.F.); National Institutes of Health grant P01-CA092625 and a Specialized Center of Research grant from the Leukemia & Lymphoma Society (both R.D.-F.). S.M. and S.C. are being supported by fellowships from Novara-AIL Onlus, Novara. L.P. is on leave from the University of Perugia Medical School.
National Institutes of Health
Authorship
Contribution: D.R., R.R., L.P., R.D.-F., R. Foà, and G.G. designed the study, interpreted data, and wrote the manuscript; D.R., M.F., and P.B. performed statistical analysis; S.R., V.S., A.B., M.C., S.C., and R. Famà performed molecular analysis; S.M. performed FISH analysis; L.D.P, F.F., L.L., and R.M. provided well characterized biological samples and clinical data; G.F., V.G., A.G., and S.D. contributed to interpretation of biological data; and D.C. was in charge of the ARMS assay.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Davide Rossi, Division of Hematology, Department of Clinical and Experimental Medicine, Amedeo Avogadro University of Eastern Piedmont, Via Solaroli 17, 28100 Novara, Italy; e-mail: rossidav@med.unipmn.it.
References
Author notes
D.R. and S.R. contributed equally to this study.
R.F. and G.G. contributed equally to this study.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal