Abstract
The retinoblastoma (Rb) tumor suppressor plays important roles in regulating hematopoiesis, particularly erythropoiesis. In an effort to understand whether Rb function can be mediated by E2F transcription factors in a BM-derived hematopoietic system in mice, we uncovered a functional synergy between Rb and E2F8 to promote erythropoiesis and to prevent anemia. Specifically, whereas Mx1-Cre–mediated inactivation of Rb or E2f8 in hematopoietic stem cells only led to mild erythropoietic defects, concomitant inactivation of both genes resulted in marked ineffective erythropoiesis and mild hemolysis, leading to severe anemia despite the presence of enhanced extramedullary erythropoiesis. Interestingly, although ineffective erythropoiesis was already present in the RbΔ/Δ mice and exacerbated in the RbΔ/Δ;E2f8Δ/Δ mice, hemolysis was exclusively manifested in the double-knockout mice. Using an adoptive transfer system and an erythroid-specific knockout system, we have shown that the synergy of Rb and E2f8 deficiency in triggering severe anemia is intrinsic to the erythroid lineage. Surprisingly, concomitant inactivation of Rb and E2f7, a close family member of E2f8, did not substantially worsen the erythropoietic defect resulted from Rb deficiency. The results of the present study reveal the specificity of E2F8 in mediating Rb function in erythropoiesis and suggest critical and overlapping roles of Rb and E2f8 in maintaining normal erythropoiesis and in preventing hemolysis.
Introduction
The role of the retinoblastoma (Rb) tumor suppressor in embryonic erythropoiesis was initially recognized when Rb-knockout embryos were analyzed.1-3 These embryos develop anemia with increased immature, nucleated RBCs. Further investigations using conditional knockout approaches, chimeric approaches, or in vitro assays led to controversy about how Rb controls hematopoiesis.4,5 The main controversy is centered on the cell-autonomous versus non–cell-autonomous action of Rb in the control of hematopoiesis. Whereas the non–cell-autonomous role of Rb in the control of hematopoiesis has been established in the placenta, fetal liver macrophages, and the BM niche, Rb plays a cell-autonomous role in stress erythropoiesis and in an in vitro erythroid-differentiation system.6-11 More recently, an erythroid-specific role of Rb in postnatal erythropoiesis has been identified.12
The role of Rb in the control of adult hematopoiesis has been established recently using conditional knockout mouse models. Inactivation of Rb specifically in hematopoietic stem cells (HSCs) led to a mild anemia, moderate splenomegaly, abnormal expansion of erythroblasts in the spleen, myeloproliferation, and suppression of B lymphopoiesis in the BM.7,10 In addition, using an erythroid-specific conditional knockout system, Sankaran et al demonstrated that Rb promotes adult erythropoiesis intrinsically by coupling cell-cycle exit with mitochondrial biogenesis.12
Previous studies have documented important roles of E2F transcription factors in mediating Rb function.13,14 In quiescent cells, Rb binds and inhibits E2Fs, preventing E2F-mediated transcriptional activation of genes required for S-phase entry and cell-cycle progression. After mitogenic signaling, quiescent cells relay a series of signaling transduction cascades that involve the activation of cyclin-dependent kinases and the phosphorylation and inactivation of Rb, leading to the subsequent release of E2Fs.15,16 In mammalian cells, there are 8 known E2f genes (E2f1-8), with the E2f3 locus encoding 2 isoforms (3a and 3b).17 Based on their structural and biochemical properties, E2Fs can be divided into 3 groups. E2F1, E2F2, and E2F3a are considered to be transcriptional activators with activities peaking during the G1/S transition when released from the Rb binding.15,16 Conversely, E2F3b, E2F4, and E2F5 are considered to be transcriptional repressors and bind to Rb or other pocket proteins (ie, p107 and p130) in quiescent cells to repress target genes. E2F6, E2F7, and E2F8 are also classified as repressors. Unlike E2F1-5, however, they do not have an Rb pocket-protein-binding domain.17,18 Therefore, their function is thought to be independent of pocket-protein binding. Although E2F6-dependent transcriptional repression is mediated through its ability to recruit the polycomb repressor complex,19 it is unclear how E2F7 and E2F8 are involved in transcriptional repression. Given the interaction between Rb pocket proteins and E2F1-5, it is expected that Rb function can be mediated through various E2F activities. Indeed, numerous studies have confirmed that E2Fs are important mediators for Rb function.20-27 In particular, Dirlam et al showed recently that the terminal differentiation defect in Rb-null erythroblasts was suppressed by the loss of E2f2.28 However, it is unclear whether non–pocket-protein-binding E2Fs, namely E2F6, E2F7, and E2F8, can interact functionally with Rb to mediate its function in vivo.
In an effort to understand whether non–pocket-protein-binding E2Fs can mediate Rb function in the hematopoietic system, we uncovered a potent functional synergy between Rb loss and E2f8 loss to induce severe anemia. Mice with HSCs deficient for both Rb and E2f8 suffer from marked ineffective erythropoiesis and mild hemolysis, leading to severe anemia despite the presence of regenerative response and active erythropoiesis. Interestingly, inactivation of E2f7, which shares all key functional domains and biochemical features with E2f8,18 does not exacerbate significantly the mild anemia observed in mice with HSCs deficient for Rb. Therefore, our data not only demonstrate the functional interaction of Rb and E2F8 to ensure normal erythropoiesis, likely by promoting erythroid terminal differentiation and maintaining erythrocyte membrane integrity, but also suggest possible roles of Rb and E2F8 in preventing hemolysis in humans.
Methods
Mice
All mouse lines used in this study have been described previously.29-32 Induction of Mx1-Cre was achieved by 3-7 IP injections of 400 μg of poly(I:C) (Sigma-Aldrich) every other day to 4-week-old mice. Mice were killed 12 weeks after poly(I:C) injection. EpoR-GFPCre–mediated knockout mice were killed at 3 months of age. All animal protocols were approved by the institutional animal care and use committee at New Jersey Medical School.
Hematologic parameters
Hematologic parameters for erythroid cells (ie, RBCs, hemoglobin, hematocrit, mean cell volume, and mean cell hemoglobin) were assessed using an automated instrument (Hemavet FS950; Drew Scientific). Hematologic findings were confirmed on peripheral blood smears by a board-certified veterinary pathologist (C.W.). Bilirubin was measured on plasma using an automated method (Olympus AU680; Beckman-Coulter).
FACS
Single-cell suspensions were labeled with surface Abs. Labeled cells were processed with a FACSCalibur flow cytometer (BD Biosciences) and analyzed using FlowJo Version 7.6 software (TreeStar). The following Abs were purchased from eBiosciences or BD Biosciences: CD2 (11-0021-81), CD3 (11-0031-81), CD4 (11-0041-81), CD5 (11-0051-81), CD8 (11-0081-81), CD11b (11-0112-81), CD71 (11-0711-85), B220 (11-0452-85), F4/80 (11-4801-82), Gr1 (11-5931-81), Ter119 (11-5921-81), Flk2 (12-1351-81), Sca 1 (45-5981-82), and c-kit (17-1171-81). For stem cell/progenitor assays, cell suspensions were first stained with lineage Abs (CD2, CD3, CD4, CD5, CD8, CD11b, Gr1, B220, and Ter119), followed by staining with Flk2, Sca 1, and c-kit. Erythroid staging based on CD71 versus forward scatter sorting was performed as described previously33 using a FACSVantage cell sorter (BD Biosciences) and DIVA Version 6 (BD Biosciences) or FlowJo Version 7.6 software (TreeStar).
Adoptive transfer and stem/progenitor assays
BM cells were collected from mice that were backcrossed to a FVB background for 2-4 generations. Either 1 × 105 (for colony-forming unit-spleen [CFU-S]) or 1-10 × 106 (for adoptive transfer) BM cells were injected intravenously into FVB mice that received 13.5 Gy of radiation. For adoptive transfer, mice were killed 3 months after transplantation. For CFU-S assays, mice were killed 8 or 12 days after transplantation and their spleens were removed and fixed in Bouin solution. The hematopoietic colony-forming assay using Methocult M3434 medium (StemCell Technologies) was performed following the directions of the manufacturer.
RBC life span
RBC life span was measured as described previously12 with minor modifications. RBCs were labeled by IV injection of N-hydroxysuccinimide biotin (Pierce; 25 μg/g body weight). Approximately 20 μL of blood was obtained via the retroorbital plexus at 3-day intervals, followed by biotinylation and incubation with FITC-Avidin (Sigma-Aldrich). The percentage of biotinylated RBCs was assessed with the FACSCalibur flow cytometer.
Erythrocyte deformability and osmotic fragility
RBCs (3 × 107) were suspended in 1 mL of 5% polyvinylpyrrolidone (Molecular Weight 360 000; Sigma-Aldrich) at a final viscosity of 29 cP and osmolality of 293 mosmol/kg H2O. Cell deformability was determined with an ektacytometer (LORCA) by calculating the elongation index.34 Osmotic fragility assay was performed as described previously.35
Cell-cycle distributions and apoptosis
Cell-cycle kinetics were assessed as described previously.12 Mice were injected intraperitoneally with 5′-bromodeoxyuridine (BrdU) at 150 mg/kg body weight. BM and spleen cells were isolated after 1 hour, fixed, and stained with CD71 and Ter119 Abs. BrdU staining and annexin V staining were performed according to the directions of the manufacturers (559619 and 550474; BD Biosciences).
Erythropoietin concentration measurement
Serum was obtained by centrifuging peripheral blood without any anticoagulant. Serum erythropoietin concentration was determined using a rodent Quantikine Epo Immunoassay kit (MEP00; R&D Systems) following the directions of the manufacturer.
Immunofluorescence staining
Immunofluorescence staining was performed as described previously36 with minor modifications. Permeabilized RBCs were stained with rhodamine-phalloidin (Molecular Probes) and counterstained with SYTOX Green (Molecular Probes) to exclude reticulocytes and leukocytes for quantifications.
Statistical analysis
Values are presented as means ± SD. Statistical significance was determined by Student t test with a significance threshold of P = .05.
Results
Concomitant inactivation of Rb and E2f8 results in severe anemia
To determine whether E2F7 and E2F8 can mediate Rb function, we inactivated Rb, E2f7, and E2f8 in HSCs using an IFN-inducible system with the Mx1-Cre allele.30 Semiquantitative PCR confirmed the efficient deletion of Rb, E2f7, and E2F8 in whole BM (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article, and data not shown). Consistent with previous studies,7,10 inactivation of Rb in HSCs (ie, RbΔ/Δ) led to mild anemia, characterized by approximately 20% reductions in RBCs, hemoglobin, and hematocrit (Table 1). Surprisingly, despite mild anemia in RbΔ/Δ mice and slight reductions of RBCs in E2f8Δ/Δ mice, concomitant inactivation of Rb and E2f8 led to severe anemia, with RBCs, hemoglobin, and hematocrit being reduced by approximately 50%. In contrast, concomitant inactivation of E2f7 did not exacerbate substantially the mild anemia resulted from Rb deficiency, suggesting that E2f8 (but not E2f7) plays a predominant role in mediating Rb function in erythropoiesis. Consistent with this notion, concomitant inactivation of E2f7 did not worsen the severe anemia in RbΔ/ΔE2f8Δ/Δ mice substantially (Table 1). In addition to reduced RBCs, hemoglobin, and hematocrit, RbΔ/Δ mice also displayed increased mean cell volume and mean cell hemoglobin, which were further increased in the RbΔ/ΔE2f8Δ/Δ mice. Interestingly, a significant reduction of mean cell hemoglobin concentrations was also observed in the RbΔ/ΔE2f8Δ/Δ mice (Table 1).
Hematological and biochemical parameters of peripheral blood
| Genotype . | RbLoxP/LoxP . | RbΔ/Δ . | E2f8Δ/Δ . | RbΔ/Δ E2f8Δ/Δ . | RbΔ/Δ E2f7Δ/Δ E2f8Δ/Δ . | RbΔ/Δ E2f7Δ/Δ . | E2f7Δ/Δ E2f8Δ/Δ . |
|---|---|---|---|---|---|---|---|
| RBCs (× 109/L) | 9.2 ± 0.3 | 6.9 ± 0.3*** | 8.6 ± 0.4** | 4.3 ± 0.2*** (***) | 4.1 ± 0.7*** (***) | 7.6 ± 0.3*** (**) | 8.6 ± 0.7* |
| Hemoglobin (g/dL) | 14.7 ± 0.8 | 11.9 ± 0.6*** | 14.4 ± 0.4 | 8.0 ± 0.2*** (***) | 8.6 ± 0.7*** (***) | 13.0 ± 0.8** (*) | 14.4 ± 0.4 |
| Hematocrit (%) | 41.4 ± 1.8 | 33.3 ± 2.4*** | 39.4 ± 3.5 | 24.5 ± 1.2*** (***) | 23.7 ± 3.3*** (***) | 37.9 ± 2.5** (**) | 38.7 ± 2.6* |
| MCV(fL) | 44.8 ± 1.7 | 48.8 ± 2.9** | 44.5 ± 1.0 | 57.6 ± 2.4*** (***) | 58.0 ± 2.7*** (***) | 50.1 ± 1.8*** | 45.0 ± 1.3 |
| MCH(fg) | 15.9 ± 1.0 | 17.4 ± 0.8** | 15.0 ± 0.8 | 18.7 ± 0.6*** (**) | 21.2 ± 2.2*** (***) | 17.2 ± 0.4* | 16.7 ± 0.9 |
| MCHC (g/dL) | 35.5 ± 1.3 | 35.7 ± 2.4 | 35.0 ± 2.3 | 32.6 ± 1.3** (*) | 36.5 ± 2.6 | 34.3 ± 1.1 | 37.1 ± 1.4* |
| RDW (%) | 17.7 ± 2.5 | 21.3 ± 2.2** | 17.2 ± 1.1 | 25.3 ± 3.2*** (*) | 27.1 ± 1.3*** (**) | ND | ND |
| Reticulocyte (× 108/L) | 2.1 ± 0.2 | 3.2 ± 0.6* | 2.6 ± 0.4 | 9.5 ± 1.5*** (**) | 8.8 ± 0.6*** (***) | ND | ND |
| nRBC (per 5000 RBCs) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 4.4 ± 0.9** (**) | ND | ND | ND |
| Erythropoietin (pg/mL) | 185.4 ± 16 | 468.6 ± 7*** | ND | 1944.2 ± 143*** (***) | 1986.0 ± 173*** (***) | ND | ND |
| Total bilirubin (mg/dL) | 0.4 ± 0 | 0.3 ± 0 | ND | 0.8 ± 0.1 | 1.2 ± 0.2 | ND | ND |
| Unconjugated bilirubin (mg/dL) | 0.3 ± 0 | 0.3 ± 0 | ND | 0.7 ± 0.1 | 1.0 ± 0.1 | ND | ND |
| Genotype . | RbLoxP/LoxP . | RbΔ/Δ . | E2f8Δ/Δ . | RbΔ/Δ E2f8Δ/Δ . | RbΔ/Δ E2f7Δ/Δ E2f8Δ/Δ . | RbΔ/Δ E2f7Δ/Δ . | E2f7Δ/Δ E2f8Δ/Δ . |
|---|---|---|---|---|---|---|---|
| RBCs (× 109/L) | 9.2 ± 0.3 | 6.9 ± 0.3*** | 8.6 ± 0.4** | 4.3 ± 0.2*** (***) | 4.1 ± 0.7*** (***) | 7.6 ± 0.3*** (**) | 8.6 ± 0.7* |
| Hemoglobin (g/dL) | 14.7 ± 0.8 | 11.9 ± 0.6*** | 14.4 ± 0.4 | 8.0 ± 0.2*** (***) | 8.6 ± 0.7*** (***) | 13.0 ± 0.8** (*) | 14.4 ± 0.4 |
| Hematocrit (%) | 41.4 ± 1.8 | 33.3 ± 2.4*** | 39.4 ± 3.5 | 24.5 ± 1.2*** (***) | 23.7 ± 3.3*** (***) | 37.9 ± 2.5** (**) | 38.7 ± 2.6* |
| MCV(fL) | 44.8 ± 1.7 | 48.8 ± 2.9** | 44.5 ± 1.0 | 57.6 ± 2.4*** (***) | 58.0 ± 2.7*** (***) | 50.1 ± 1.8*** | 45.0 ± 1.3 |
| MCH(fg) | 15.9 ± 1.0 | 17.4 ± 0.8** | 15.0 ± 0.8 | 18.7 ± 0.6*** (**) | 21.2 ± 2.2*** (***) | 17.2 ± 0.4* | 16.7 ± 0.9 |
| MCHC (g/dL) | 35.5 ± 1.3 | 35.7 ± 2.4 | 35.0 ± 2.3 | 32.6 ± 1.3** (*) | 36.5 ± 2.6 | 34.3 ± 1.1 | 37.1 ± 1.4* |
| RDW (%) | 17.7 ± 2.5 | 21.3 ± 2.2** | 17.2 ± 1.1 | 25.3 ± 3.2*** (*) | 27.1 ± 1.3*** (**) | ND | ND |
| Reticulocyte (× 108/L) | 2.1 ± 0.2 | 3.2 ± 0.6* | 2.6 ± 0.4 | 9.5 ± 1.5*** (**) | 8.8 ± 0.6*** (***) | ND | ND |
| nRBC (per 5000 RBCs) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 4.4 ± 0.9** (**) | ND | ND | ND |
| Erythropoietin (pg/mL) | 185.4 ± 16 | 468.6 ± 7*** | ND | 1944.2 ± 143*** (***) | 1986.0 ± 173*** (***) | ND | ND |
| Total bilirubin (mg/dL) | 0.4 ± 0 | 0.3 ± 0 | ND | 0.8 ± 0.1 | 1.2 ± 0.2 | ND | ND |
| Unconjugated bilirubin (mg/dL) | 0.3 ± 0 | 0.3 ± 0 | ND | 0.7 ± 0.1 | 1.0 ± 0.1 | ND | ND |
Shown are mean values ± SD. n ≥ 3 per genotypic groups except for total bilirubin and unconjugated bilirubin assays (n = 2). Asterisks represent statistical comparisons to RbLoxP/LoxP mice, whereas asterisks in parentheses represent statistical comparisons to RbΔ/Δ mice.
nRBC indicates nucleated RBC; RDW, red cell distribution width; and ND, not determined.
P < .05.
P < .01.
P < .001.
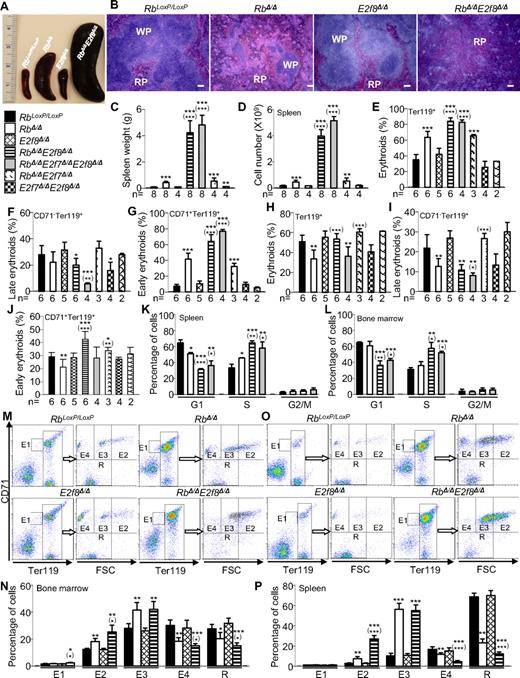
As described previously,7,10 inactivation of Rb in HSCs led to moderate splenomegaly (Figure 1A). Although there were no significant increases of spleen weights in the E2f8Δ/Δ mice, the spleens of RbΔ/ΔE2f8Δ/Δ mice were enormous, being increased by approximately 50-fold compared with those of control mice (Figure 1C). Conversely, RbΔ/ΔE2f7Δ/Δ mice had spleens similar in size to the RbΔ/Δ mice (Figure 1C), further supporting a predominant role of E2f8 in mediating Rb function in erythropoiesis. As expected, the numbers of splenocytes in RbΔ/ΔE2f8Δ/Δ mice were also increased dramatically (Figure 1D). However, there were no significant differences in BM cellularity in any of the genotypic groups (supplemental Figure 2).
Mx1-Cre–mediated inactivation of Rb and E2f8 in HSCs results in enhanced but ineffective erythropoiesis. (A) Representative pictures of whole spleens of mice with the indicated genotypes. (B) Representative pictures of H&E-stained sections of spleens from mice with indicated genotypes. The scale bar indicates 100 μm. (C-D) Spleen weights (C) and spleen cell numbers (D) of mice with the indicated genotypes. (E-J) Flow cytometric analysis for total erythroid cells, late erythroid cells, and early erythroid cells in spleens (E-G) and BM (H-J). (K-L) Cell-cycle distributions based on a BrdU incorporation assay for early erythroid cells (CD71+Ter119+) in spleens (K) and BM (L) from mice with the indicated genotypes (n = 3 mice/genotypic group). (M-P) Erythroid staging by flow cytometric analysis of Ter119+ BM (M-N) and spleen cells (O-P) sorted by CD71 and cell size (forward scatter, FSC). (M,O) Representative flow cytometric profiles. (N,P) Percentages of different erythroid subpopulations were normalized to total Ter119+ cells (n = 4 mice/genotypic group). In all figures, asterisks indicate statistical comparisons with control (RbLoxP/LoxP) mice and asterisks in parentheses indicate statistical comparisons with Rb-knockout mice as follows: *P < .05; **P < .01; and ***P < .001.
Mx1-Cre–mediated inactivation of Rb and E2f8 in HSCs results in enhanced but ineffective erythropoiesis. (A) Representative pictures of whole spleens of mice with the indicated genotypes. (B) Representative pictures of H&E-stained sections of spleens from mice with indicated genotypes. The scale bar indicates 100 μm. (C-D) Spleen weights (C) and spleen cell numbers (D) of mice with the indicated genotypes. (E-J) Flow cytometric analysis for total erythroid cells, late erythroid cells, and early erythroid cells in spleens (E-G) and BM (H-J). (K-L) Cell-cycle distributions based on a BrdU incorporation assay for early erythroid cells (CD71+Ter119+) in spleens (K) and BM (L) from mice with the indicated genotypes (n = 3 mice/genotypic group). (M-P) Erythroid staging by flow cytometric analysis of Ter119+ BM (M-N) and spleen cells (O-P) sorted by CD71 and cell size (forward scatter, FSC). (M,O) Representative flow cytometric profiles. (N,P) Percentages of different erythroid subpopulations were normalized to total Ter119+ cells (n = 4 mice/genotypic group). In all figures, asterisks indicate statistical comparisons with control (RbLoxP/LoxP) mice and asterisks in parentheses indicate statistical comparisons with Rb-knockout mice as follows: *P < .05; **P < .01; and ***P < .001.
Histologic examination of H&E-stained spleen sections indicated that, unlike spleens from control mice and RbΔ/Δ mice, which consisted of red pulp and white pulp, spleens from RbΔ/ΔE2f8Δ/Δ mice lacked white pulp and were almost completely filled with erythroid progenitors (Figure 1B), suggesting the presence of massive extramedullary erythropoiesis. Flow cytometric analyses revealed enormous increases in early erythroid cells (CD71+Ter119+) and decreases in late erythroid cells (CD71−Ter119+) in the spleens of RbΔ/ΔE2f8Δ/Δ mice (Figure 1E-G). Consistent with previous studies,7,10 RbΔ/Δ mice showed a decrease in erythroid cells (both early and late) in the BM (Figure 1H-J). Interestingly, although there was no significant difference in the number of total erythroid cells in the BM of RbΔ/ΔE2f8Δ/Δ mice compared with those from control mice, there was a decrease in late erythroid cells and an increase in early erythroid cells (Figure 1H-J). Because there was a substantial myeloid expansion in the BM of the RbΔ/ΔE2f8Δ/Δ mice (supplemental Figure 4), the increased percentage of early erythroid cells in the BM, coupled with the substantial increases of early erythroid cells in the spleen, suggests active erythroid regeneration in response to the anemia. Consistent with this notion, RbΔ/ΔE2f8Δ/Δ mice had a 10-fold increase in erythropoietin levels, a 4.5-fold increase in circulating reticulocytes, and increased nucleated RBCs, anisocytosis (increased RBC distribution width), and polychromasia in their peripheral blood (supplemental Figure 5 and Table 1). In addition, BrdU incorporation analysis of early erythroid cells of spleens and BM revealed significantly higher percentages of S-phase cells and significantly lower percentages of G1-phase cells in RbΔ/ΔE2f8Δ/Δ mice than in control or RbΔ/Δ mice (Figure 1K-L). These data suggest that early erythroid cells in RbΔ/ΔE2f8Δ/Δ mice were highly proliferating. Conversely, early erythroid cells from both spleens and BM showed similar levels of apoptosis among the different genotypic groups (supplemental Figure 3), suggesting that the expansion of early erythroid cells likely resulted from increased proliferation instead of decreased apoptosis.
Impaired erythroid differentiation in RbΔ/Δ mice and RbΔ/ΔE2f8Δ/Δ mice
Considering the important role of Rb in erythropoiesis6,12 and the severe anemia in RbΔ/ΔE2f8Δ/Δ mice, we investigated whether ineffective erythropoiesis can contribute to the severe anemia using a recently established erythroid-staging system33 to differentiate Ter119+ cells into 5 subpopulations sorted based on Ter119, CD71, and cell size (forward scatter). Similar to the previous study,33 our sorted subpopulations represent erythroid cells at different developmental stages with reasonably good purities (supplemental Figure 6). Using this system, we found that the E3 and E2 subpopulations representing late-stage erythroid precursors were increased significantly in RbΔ/Δ mice and further enriched in those from RbΔ/ΔE2f8Δ/Δ mice (Figure 1M-P and supplemental Figure 6). In contrast, subpopulation E4 (predominantly reticulocytes) and R (almost exclusively RBCs) were reduced significantly in both the BM and spleens from RbΔ/Δ mice and further reduced in those from RbΔ/ΔE2f8Δ/Δ mice. The significant enrichment of late-stage erythroid precursors and the significant reduction of reticulocytes and RBCs in both spleens and BM support the idea that RbΔ/ΔE2f8Δ/Δ mice suffer from defects in erythroid terminal differentiation, leading to marked ineffective erythropoiesis that significantly contributes to the severe anemia. Interestingly, unlike in humans, where ineffective erythropoiesis is often associated with elevated apoptosis, erythroid precursors in the RbΔ/ΔE2f8Δ/Δ mice appeared to have normal apoptosis rates (supplemental Figure 3), reminiscent of the Rb-knockout mouse model in which ineffective erythropoiesis is present without measurable changes in apoptosis rates.12
Hemolysis in RbΔ/ΔE2f8Δ/Δ mice
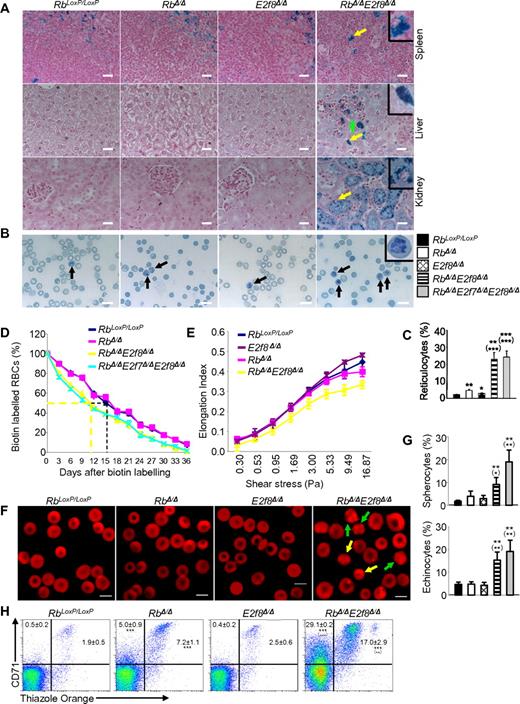
To evaluate whether RbΔ/ΔE2f8Δ/Δ mice experience intravascular hemolysis, we performed Prussian blue staining on paraffin-embedded tissue sections to assess iron deposits. As expected, spleen sections from mice with all genotypic groups contained Prussian blue–positive cells representing macrophages and reflecting baseline hemolysis (Figure 2A). There was no obvious Prussian blue staining in livers or kidneys from control, RbΔ/Δ, or E2f8Δ/Δ mice. In sharp contrast, RbΔ/ΔE2f8Δ/Δ mice displayed marked staining in renal proximal tubular cells, moderate staining in liver Kupffer cells, and weak staining in hepatocytes surrounding hematopoietic foci. Bilirubin, particularly unconjugated bilirubin, was moderately increased in the sera of RbΔ/ΔE2f8Δ/Δ mice and RbΔ/ΔE2f7Δ/ΔE2f8Δ/Δ mice compared with those of control mice or RbΔ/Δ mice (Table 1), suggesting the presence of mild hemolysis. Consistent with this notion, peripheral RBCs from RbΔ/ΔE2f8Δ/Δ mice had a 4.5-fold increase in circulating reticulocytes, moderately shortened life spans (half-lives being reduced from approximately 16 days to approximately 11 days), slightly reduced deformability, and moderately increased osmotic fragility (Figure 2B-E, supplemental Figure 7, and Table 1). Morphologically, both spherocytes and echinocytes were increased in these mice (Figure 2F-G). These data suggest that inactivation of Rb and E2f8 synergizes to trigger mild hemolysis.
Mx1-Cre–mediated inactivation of Rb and E2f8 in HSCs leads to hemolysis. (A) Prussian blue staining of tissue sections from mice with the indicated genotypes. Yellow arrows indicate a positive macrophage in the spleen, a positive Kupffer cell in the liver, and a positive proximal tubular cell in the kidney, whereas the green arrow indicates a hepatocyte with weak Prussian blue staining. Insets represent enlarged images of representative positive cells. Scale bar indicates 20 μm. (B) Representative images of new methylene blue staining of blood smears prepared from mice with the indicated genotypes. Black arrows show typical reticulocytes on blood smears. The inset shows an enlarged image of a representative reticulocytes. Scale bar indicates 10 μm. (C) Percentages of reticulocytes quantified from panel B. A minimum of 200 RBCs were counted for analysis (n ≥ 3 mice/genotypic group). (D) RBC life spans were assessed by biotin labeling and cytometric analysis (n ≥ 3 mice/genotypic group). (E) RBC deformability was assessed by ektacytometry (n ≥ 3 mice/genotypic group). (F) Representative confocal images of peripheral blood stained with rhodamine-phalloidin. Yellow arrows show spherocytes and green arrows show echinocytes. (G) Quantifications of data from panel F for abnormal erythrocytes (spherocytes and echinocytes). Reticulocytes and leukocytes were excluded from the quantification by counterstaining the peripheral blood with SYTOX Green. Images were acquired on a Zeiss LSM 510 confocal microscope and analyzed with LSM Image Browser Version 4.2 (Carl Zeiss). Scale bar indicates 5 μm. A minimum of 200 cells were counted for analysis (n = 3 mice/genotypic group). (H) Representative flow cytometric profiles using CD71 Abs and Thiazole Orange and percentages of reticulocytes (CD71+TO+) and CD71+ erythrocytes (CD71+TO−) in the peripheral blood. Numbers are mean percentages ± SD (n ≥ 3 mice/genotypic group).
Mx1-Cre–mediated inactivation of Rb and E2f8 in HSCs leads to hemolysis. (A) Prussian blue staining of tissue sections from mice with the indicated genotypes. Yellow arrows indicate a positive macrophage in the spleen, a positive Kupffer cell in the liver, and a positive proximal tubular cell in the kidney, whereas the green arrow indicates a hepatocyte with weak Prussian blue staining. Insets represent enlarged images of representative positive cells. Scale bar indicates 20 μm. (B) Representative images of new methylene blue staining of blood smears prepared from mice with the indicated genotypes. Black arrows show typical reticulocytes on blood smears. The inset shows an enlarged image of a representative reticulocytes. Scale bar indicates 10 μm. (C) Percentages of reticulocytes quantified from panel B. A minimum of 200 RBCs were counted for analysis (n ≥ 3 mice/genotypic group). (D) RBC life spans were assessed by biotin labeling and cytometric analysis (n ≥ 3 mice/genotypic group). (E) RBC deformability was assessed by ektacytometry (n ≥ 3 mice/genotypic group). (F) Representative confocal images of peripheral blood stained with rhodamine-phalloidin. Yellow arrows show spherocytes and green arrows show echinocytes. (G) Quantifications of data from panel F for abnormal erythrocytes (spherocytes and echinocytes). Reticulocytes and leukocytes were excluded from the quantification by counterstaining the peripheral blood with SYTOX Green. Images were acquired on a Zeiss LSM 510 confocal microscope and analyzed with LSM Image Browser Version 4.2 (Carl Zeiss). Scale bar indicates 5 μm. A minimum of 200 cells were counted for analysis (n = 3 mice/genotypic group). (H) Representative flow cytometric profiles using CD71 Abs and Thiazole Orange and percentages of reticulocytes (CD71+TO+) and CD71+ erythrocytes (CD71+TO−) in the peripheral blood. Numbers are mean percentages ± SD (n ≥ 3 mice/genotypic group).
In addition to a synergistic increase in reticulocytes (CD71+TO+) from the peripheral blood of RbΔ/ΔE2f8Δ/Δ mice compared with those of RbΔ/Δ mice and E2f8Δ/Δ mice, we also observed a synergistic increase in the CD71+TO− population, which represents enucleated RBCs with high levels of CD71 (Figure 2H). Because down-regulation of the transferrin receptor (CD71) is a critical step for erythrocyte membrane remodeling as reticulocytes mature to enucleated RBCs,37 these data suggest compromised membrane remodeling process in the RbΔ/ΔE2f8Δ/Δ mice. Interestingly, levels of 6 discernible major membrane proteins seemed to be comparable among ghosts collected from various genotypic groups, although 2 unknown bands appeared to be more intense in the RbΔ/ΔE2f8Δ/Δ mice (supplemental Figure 6).
Depletion of HSCs/hematopoietic progenitor cells in the BM of RbΔ/ΔE2f8Δ/Δ mice
As observed in many anemic mouse models in which the spleen is the major source of increased RBC production because of limited capacity of BM expansion, HSCs and progenitors are significantly reduced in the BM and increased in the spleens of RbΔ/ΔE2f8Δ/Δ mice (Figure 3A-B). Consistent with these data, significant decreases of BM in vivo, day 8 and 12 CFU-S, and expansions of lineage-committed progenitors (CFU-GEMM, CFU-GM, and BFU-E) in the spleens were also observed in these mice (Figure 3C-G). Consistent with myeloproliferation and expansions of early erythroid cells in the BM (Figure 1 and supplemental Figure 4), both the BFU-E and CFU-GM populations were significantly increased in the BM of RbΔ/ΔE2f8Δ/Δ mice (Figure 3H). These data suggest that in RbΔ/ΔE2f8Δ/Δ mice, HSCs in the BM are mobilized to the spleen to differentiate into lineage-committed progenitors in response to severe anemia.
Depletion of HSCs in the BM of RbΔ/ΔE2f8Δ/Δ mice. (A-B) The absolute numbers of phenotypic stem cells (Lin−c-kit+Sca-1+ [LKS+]), progenitors (Lin−c-kit+Sca-1− [LKS−]), and long-term stem cells (Flk2−Lin−c-kit+Sca-1+) in the BM (A) and spleens (B) were estimated by flow cytometric analysis. Values above each bar in panel B represent the fold increases compared with control mice. (C-F) Photographs (C-D) and numbers (E-F) of CFU-S colonies from mice with the indicated genotypes. (G-H) Numbers of CFU-GEMM, CFU-GM, and BFU-E in the spleens (G) and BM (H) of mice with the indicated genotypes.
Depletion of HSCs in the BM of RbΔ/ΔE2f8Δ/Δ mice. (A-B) The absolute numbers of phenotypic stem cells (Lin−c-kit+Sca-1+ [LKS+]), progenitors (Lin−c-kit+Sca-1− [LKS−]), and long-term stem cells (Flk2−Lin−c-kit+Sca-1+) in the BM (A) and spleens (B) were estimated by flow cytometric analysis. Values above each bar in panel B represent the fold increases compared with control mice. (C-F) Photographs (C-D) and numbers (E-F) of CFU-S colonies from mice with the indicated genotypes. (G-H) Numbers of CFU-GEMM, CFU-GM, and BFU-E in the spleens (G) and BM (H) of mice with the indicated genotypes.
Erythroid-intrinsic role of Rb and E2f8 in maintaining normal erythropoiesis and preventing hemolysis
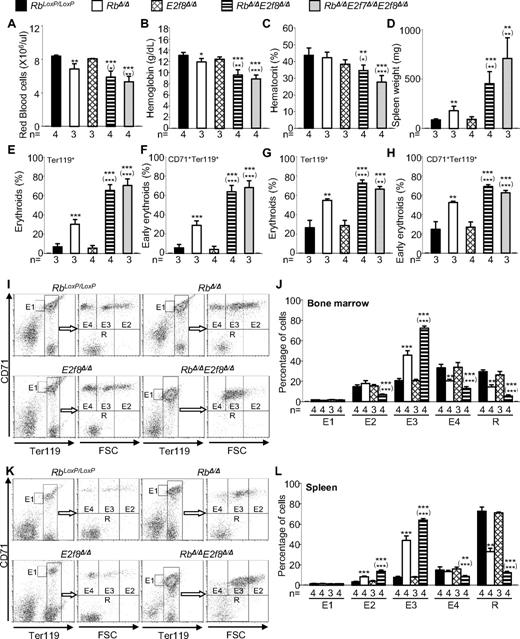
Our data show that inactivation of E2f8 synergizes with Rb deficiency to induce severe anemia. To determine whether the role of Rb and E2f8 in regulating erythropoiesis is cell autonomous, we transplanted BM cells into lethally irradiated wild-type mice. Because inactivation of Rb and E2f8 causes HSC depletion in the BM, lethally irradiated mice receiving the standard number (2 × 106) of RbΔ/ΔE2f8Δ/Δ BM cells failed to survive. We overcame this problem by injecting either more RbΔ/ΔE2f8Δ/Δ donor cells (6-10 × 106), or the standard number (2 × 106) of Mx1-Cre+/−RbLoxP/LoxPE2f8LoxP/LoxP BM cells followed by poly(I:C) injection 35 days after transplantation to inactivate Rb and E2f8. Both approaches yielded similar results. Specifically, mice receiving RbΔ/ΔE2f8Δ/Δ BM cells recapitulated all erythropoietic defects observed in mice with Mx1-Cre–mediated inactivation of Rb and E2f8, including severe anemia, profound splenomegaly, and enormous expansion of early erythroid cells (Figure 4). These data suggest that the functional interaction of Rb and E2f8 in erythropoiesis is cell autonomous. In addition, inactivation of Rb and E2f8 specifically in the erythroid lineage by the EpoR-GFPCre allele29 also recapitulated the synergy of Rb and E2f8 loss in triggering severe anemia that resulted from both ineffective erythropoiesis and hemolysis (Figure 5A-H and supplemental Figure 9). These data support the idea that the collaborative role of Rb and E2f8 in maintaining normal erythropoiesis and preventing hemolysis is erythroid intrinsic.
Lethally irradiated mice harboring HSCs deficient for Rb and E2f8 suffer from severe anemia. (A-D) Peripheral blood RBC numbers (A), hemoglobin (B), hematocrit (C), and spleen weights (D) of mice with the indicated genotypes. (E-J) Flow cytometric analysis of spleens (E-F) and BM (G-H) using lineage-specific cell-surface markers. Data labeled “post” were from mice that received BM donor cells harvested from mice that were injected with poly(I:C), whereas data labeled “+35” were from mice that received BM donor cells harvested from mice that had not been injected with poly(I:C). In the latter case, recipient mice were injected with poly(I:C) 35 days after transplantation.
Lethally irradiated mice harboring HSCs deficient for Rb and E2f8 suffer from severe anemia. (A-D) Peripheral blood RBC numbers (A), hemoglobin (B), hematocrit (C), and spleen weights (D) of mice with the indicated genotypes. (E-J) Flow cytometric analysis of spleens (E-F) and BM (G-H) using lineage-specific cell-surface markers. Data labeled “post” were from mice that received BM donor cells harvested from mice that were injected with poly(I:C), whereas data labeled “+35” were from mice that received BM donor cells harvested from mice that had not been injected with poly(I:C). In the latter case, recipient mice were injected with poly(I:C) 35 days after transplantation.
EpoR-GFPCre–mediated inactivation of Rb and E2f8 in the erythroid compartment results in severe anemia and enhanced but ineffective erythropoiesis. (A-D) Peripheral blood RBC numbers (A), hemoglobin (B), hematocrit (C), and spleen weights (D) of mice with the indicated genotypes. (E-K) Flow cytometric analysis of spleens (E-F) and BM (G-K) using lineage-specific cell-surface markers. (I-L) Erythroid staging by flow cytometric analysis of Ter119+ BM (I-J) and spleen cells (K-L) sorted by CD71 and cell size (forward scatter, FSC). (I,K) Representative flow cytometric profiles. (J,L) Percentages of different erythroid subpopulations normalized to total Ter119+ cells.
EpoR-GFPCre–mediated inactivation of Rb and E2f8 in the erythroid compartment results in severe anemia and enhanced but ineffective erythropoiesis. (A-D) Peripheral blood RBC numbers (A), hemoglobin (B), hematocrit (C), and spleen weights (D) of mice with the indicated genotypes. (E-K) Flow cytometric analysis of spleens (E-F) and BM (G-K) using lineage-specific cell-surface markers. (I-L) Erythroid staging by flow cytometric analysis of Ter119+ BM (I-J) and spleen cells (K-L) sorted by CD71 and cell size (forward scatter, FSC). (I,K) Representative flow cytometric profiles. (J,L) Percentages of different erythroid subpopulations normalized to total Ter119+ cells.
Discussion
Using conditional knockout mouse models, in the present study, we uncovered a potent and erythroid-intrinsic synergy of inactivation of E2f8 and Rb to trigger severe anemia. Our data represent the first evidence that a specific E2F family member that does not have a consensus pocket-protein–binding domain can functionally interact with a pocket protein in vivo. We found that although deletion of Rb or E2f8 in HSCs leads to mild erythropoietic defects (for Rb) or almost no defect (for E2f8), concomitant inactivation of both genes exacerbated the erythropoietic defect resulted from Rb deficiency significantly, leading to severe anemia. We also showed that the severe anemia in the RbΔ/ΔE2f8Δ/Δ mice resulted from both profound ineffective erythropoiesis and mild hemolysis. Whereas ineffective erythropoiesis is already present in RbΔ/Δ mice but is much more profound in RbΔ/ΔE2f8Δ/Δ mice, hemolysis is only present when both Rb and E2f8 are inactivated. RbΔ/ΔE2f8Δ/Δ mice are responsive to the anemia, as evidenced by the enhanced but still ineffective erythropoiesis and increased circulating reticulocyte counts. However, the underlying ineffective erythropoiesis and persistent mild hemolysis still render the mice severely anemic. Therefore, the steady reticulocytosis observed in the RbΔ/ΔE2f8Δ/Δ mice (Table 1) is not an indicator of effective erythropoiesis, but rather is a reflection of regenerative erythropoiesis, the premature release of immature reticulocytes to the peripheral circulation to compensate for the severe anemia, and the longer time for such reticulocytes to mature into RBCs in the peripheral circulation.
During their life span, RBCs face strong circulatory shearing forces through circulation and microcirculation. Therefore, they must be flexible to pass safely through blood vessels and capillaries. The deformability and durability of RBCs are largely controlled by membrane skeletal proteins. Mutations causing defects in one or more membrane skeletal proteins have often been found in patients with hemolytic anemia.38 In the last decade, many mouse models for hemolytic anemia with spontaneous mutations or targeted disruptions of genes encoding membrane skeletal proteins have been generated. Data from these models further support the important role of erythrocyte membrane skeletal proteins.38 Hemolysis may also arise from impaired glucose metabolism, hemoglobin synthesis, or defective erythrocyte membrane permeability.39-42 However, it remains largely unknown how these critical proteins are regulated. In the present study, we have shown that inactivation of Rb and E2f8 in erythroid cells synergizes to trigger hemolysis (Figure 2). Increased osmotic fragility, impaired deformability, and abnormal morphologies of RbΔ/ΔE2f8Δ/Δ erythrocytes suggest that the increased hemolysis is possibly because of impaired RBC membrane integrity. In addition, defective down-regulation of CD71 was also found in the RbΔ/ΔE2f8Δ/Δ reticulocytes, suggesting a membrane-remodeling defect during the reticulocyte maturation process. Many factors can contribute to erythrocyte membrane integrity, such as depleted cytoskeletal membrane proteins, abnormal partitioning of such proteins into various protein complexes, or defective membrane physiology such as a sodium leak or other ion channel defects. Given the important role of Rb in the oxidative stress response4 and mitochondrial biogenesis,12 increased oxidative stress and/or abnormal mitochondrial activities may also be the cause of hemolysis in RbΔ/ΔE2f8Δ/Δ mice.
As the 2 recently identified E2F transcriptional repressors, E2F7 and E2F8 share several unique features that distinguish them from other members of the E2F family.18 Consistent with their structural and biochemical similarities, E2F7 and E2F8 are functionally redundant during early mouse development, because E2f7−/−E2f8−/− embryos die in utero even though E2f7- or E2f8-knockout mice are viable.31 In the present study, we identified a unique synergy between loss of E2f8 (but not E2f7) and Rb deficiency to induce severe anemia. It will be interesting to know whether the difference between E2F7 and E2F8 in mediating Rb function is because of their differential promoter regulations or their different protein identities. Because E2F8 lacks a Rb pocket-protein–binding domain,18 its functional interaction with Rb is likely independent of pocket-protein binding.
Considering the corepressor role of Rb and the transcriptional repressor role of E2F8, we speculate that the potent synergy is most likely because of transcriptional deregulation or derepression of critical E2F targets gene(s) involved in maintaining normal erythropoiesis and in preventing hemolysis. Whereas the precise molecular mechanism underlying the synergy remains to be delineated, we propose 2 possible molecular mechanisms to explain how E2F8 can interact functionally with Rb to prevent target gene deregulation and severe anemia. The first possibility is the direct and overlapping down-regulation of critical targets by Rb and E2F8. In this context, Rb and E2F8 may repress either the same or different critical targets that are redundant to prevent anemia. As a transcriptional corepressor, in the absence of E2F8, Rb can impose active transcriptional repression of target genes by its association with repressor complexes. Conversely, in the absence of Rb, as a transcriptional repressor, E2F8 can maintain active repression. In the absence of both genes, however, critical target(s) would be deregulated. The fact that Rb can interact with various hematopoietic/erythroid transcription factors, such as GATA-1, PU1, and EKLF,43-45 suggests that Rb may exert its corepressor function by repressing critical targets for erythropoiesis.
The second possibility is the involvement of an activator E2F (E2F1-3) that regulates target genes important for maintaining normal erythropoiesis and for preventing hemolysis. In this context, Rb can bind to the activator E2F and prevent E2F-mediated transcriptional activation of target genes. As a transcriptional repressor, E2F8 can either repress the activator E2F or compete with it for E2F-binding sites of target gene promoters. In the absence of Rb, whereas the activator E2F is expected to be free from Rb binding and inhibition, E2F8 can still repress the activator E2F or occupy its E2f-binding sites of target gene promoters to maintain relatively normal regulation of the target genes. In the absence of E2f8, Rb binding and inhibition of the activator E2F may be sufficient to prevent aberrant activation of E2F target genes. However, in the absence of both genes, the activator E2F can be deregulated, leading to aberrant activation of target genes. The involvement of E2F2 in mediating Rb function in embryonic erythropoiesis28 raises an intriguing possibility that E2F2 is a relevant activator E2F responsible for the synergy in adult erythropoiesis. Consistent with this possibility, E2F2 has been linked functionally to GATA-1 and EKLF, 2 important erythroid transcription factors that are critical for erythroid terminal differentiation28,43,44 and that are implicated in hemolysis.46,47
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Gustavo Leone and Alain deBruin for providing E2f7LoxP/LoxP and E2f8LoxP/LoxP mice before their publication; Drs Ursula Klingmueller and James D. Engel for providing EpoR-GFPCre mice; Dr Anton Berns for providing RbLoxP/LoxP mice; Dr Mohandas Narla for critically reviewing the manuscript; and Drs David Lagunoff, Dianne Pulte, Joseph Bertino, Pranela Rameshwar, Edwin Deitch, Michael Mathews, Tulin Budak-Alpdogan, Limin Shu, and Dan Li for technical assistance.
This work was supported by grants from the National Institutes of Health, the Leukemia Research Foundation, and the University of Medicine and Dentistry of New Jersey Foundation (to L.W). T.H. is a postdoctoral fellow of the New Jersey Commission on Cancer Research.
National Institutes of Health
Authorship
Contribution: T.H. designed and performed the experiments and wrote and edited the manuscript; S.G. performed and assisted with the experiments, and edited the manuscript; C.S. assisted with the experiments; C.W. and V.C. designed the experiments and edited the manuscript; and L.W. designed the experiments and wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lizhao Wu, New Jersey Medical School–University Hospital Cancer Center, University of Medicine and Dentistry of New Jersey, 205 South Orange Ave, Newark, NJ 07103; e-mail: wuli@umdnj.edu.

![Figure 3. Depletion of HSCs in the BM of RbΔ/ΔE2f8Δ/Δ mice. (A-B) The absolute numbers of phenotypic stem cells (Lin−c-kit+Sca-1+ [LKS+]), progenitors (Lin−c-kit+Sca-1− [LKS−]), and long-term stem cells (Flk2−Lin−c-kit+Sca-1+) in the BM (A) and spleens (B) were estimated by flow cytometric analysis. Values above each bar in panel B represent the fold increases compared with control mice. (C-F) Photographs (C-D) and numbers (E-F) of CFU-S colonies from mice with the indicated genotypes. (G-H) Numbers of CFU-GEMM, CFU-GM, and BFU-E in the spleens (G) and BM (H) of mice with the indicated genotypes.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/19/10.1182_blood-2011-10-388231/4/m_zh89991290160003.jpeg?Expires=1763481433&Signature=xtDi7~5lTntw~6d9PX7LpafYZDDl9sDBGD8jTss~oVFkdlm2cU3YXYgH2QrAdmlOxM6za6LUBr~5ukbpvDDNc3Tbadj6w~kJ9WuucgTOJSF2nEZuHoyoBV06PPQQXCdNw4DMy7XfDOb4A6yyyC1GvFBegtYOjLAuUlzbkJOmAZyNtGFL0HFRyaJePQa-Uq5dKyXhEGs5JttmqNIdaJMhy-U5vgu1ZQP4becZDaTJHoHQ-sOmokhBG7~-hrC5fWUuwM4E77YSCVOuwnyXpL8ETbeX5VXbmPouORxJapboL1ZBCbpcvqhsrMzdOaXZngBhgNaxNqyUGNaSleWNjoNZ4w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal