Abstract
The purpose of this study was to define the risk of progression and survival of patients with smoldering Waldenström macroglobulinemia (SWM). SWM is defined clinically as having a serum monoclonal IgM protein ≥ 3 g/dL and/or ≥ 10% bone marrow lymphoplasmacytic infiltration but no evidence of end-organ damage (anemia, constitutional symptoms, hyperviscosity, lymphadenopathy, or hepatosplenomegaly). We searched a computerized database and reviewed the medical records of all patients at Mayo Clinic who fulfilled the criteria of SWM between 1974 and 1995. During 285 cumulative person-years of follow-up of the 48 patients with SWM (median, 15.4 years), 34 (71%) progressed to symptomatic Waldenström macroglobulinemia (WM) requiring treatment, one to primary amyloidosis, and one to lymphoma (total, 75%). The cumulative probability of progression to symptomatic WM, amyloidosis, or lymphoma was 6% at 1 year, 39% at 3 years, 59% at 5 years, and 68% at 10 years. The major risk factors for progression were percentage of lymphoplasmacytic cells in the bone marrow, size of the serum M-spike, and the hemoglobin value. Patients with SWM should be followed and not treated until symptomatic WM develops. Treatment on a clinical trial for those at greatest risk of progression should be considered.
Introduction
In 1944, Jan Waldenström described 2 patients with a large homogeneous γ-globulin component with a sedimentation coefficient of 19S to 20S and a molecular weight of approximately 1 million.1 This serum globulin was subsequently identified as an immunoglobulin and designated as IgM. The 2 patients had oronasal bleeding (now recognized as a manifestation of hyperviscosity), normochromic anemia, elevation of the erythrocyte sedimentation rate, thrombocytopenia, low serum fibrinogen, and lymphadenopathy.1 This disease entity, subsequently referred to as Waldenström macroglobulinemia (WM), is characterized by a clonal proliferation of lymphoplasmacytic cells that secrete an IgM monoclonal protein.
WM is now recognized as a distinct clinical entity defined by the presence of an IgM monoclonal gammopathy (regardless of the size of the M protein), more than or equal to 10% bone marrow infiltration (usually intertrabecular) by small lymphocytes that exhibit plasmacytoid or plasma cell differentiation and a typical immunophenotype (eg, surface IgM+, CD5+/−, CD10−, CD19+, CD20+, CD23−) and exclusion of other lymphoproliferative disorders, including chronic lymphocytic leukemia and lymphoma.2,3 The clinical features include constitutional symptoms consisting of weakness or fatigue from anemia, fever, night sweats, or weight loss.4
The consensus panel from the Second International Workshop on WM recommended that initiation of therapy was appropriate for patients with constitutional symptoms (recurrent fever, night sweats, fatigue, or weight loss), progressive symptomatic lymphadenopathy or splenomegaly, hemoglobin ≤ 10 g/dL or platelets < 100 × 109/L, or complications, such as hyperviscosity, severe sensory motor peripheral neuropathy, systemic amyloidosis, renal insufficiency, or symptomatic cryoglobulinemia.4
Smoldering WM (SWM) is a poorly described asymptomatic disorder with a high risk of progressing to symptomatic WM requiring treatment. It is defined by the presence of serum IgM ≥ 3 g/dL and/or ≥ 10% bone marrow lymphoplasmacytic infiltration but no evidence of end-organ damage (eg, symptomatic anemia, constitutional symptoms, hyperviscosity, lymphadenopathy, or hepatosplenomegaly) that can be attributed to a plasma cell proliferative disorder.2,3 Biologically, most patients with SWM have an IgM monoclonal gammopathy of undetermined significance (MGUS) but have a greater risk for progression to symptomatic WM.
We report here on the prognosis of SWM and risk factors for progression to WM in a well-defined cohort of patients for whom long-term follow-up data were available and in whom the disease was clearly defined.
Methods
Subjects
After the study was approved by the Mayo Clinic Institutional Review Board, we searched a computerized database and reviewed the medical records of all patients who had been seen at Mayo Clinic within 30 days after detection of an IgM monoclonal protein of 3 g/dL or more or a bone marrow containing 10% or more lymphoplasmacytic cells from January 1, 1974, to December 31, 1995, allowing for a potential 15-year follow-up. Patients presenting with symptomatic WM, lymphoma, chronic lymphocytic leukemia, or primary (AL) amyloidosis were excluded as were patients who had previously received chemotherapy.
Data collection
Follow-up included a review of the medical records of all 48 patients and of death certificates for those who had died. Patients were sent a letter of inquiry or contacted by telephone if they had not visited Mayo Clinic in the preceding year. The primary end point was progression to symptomatic WM requiring chemotherapy or AL amyloidosis consisting of positive results on Congo red staining and characteristic clinical features of systemic amyloidosis requiring therapy.
Statistical analysis
Progression was calculated by both the cumulative probability and the cumulative incidence of progression. The cumulative probability was calculated by a Kaplan-Meyer analysis,5 in which data from patients who had died were censored and curves were compared by the log-rank test.6 The cumulative incidence curve, which accounted for death as a competing risk, was compared with the method of Gooley et al.7 Effects of potential risk factors on progression were examined in a Cox proportional-hazards model.8 The risk of progression to symptomatic WM or amyloidosis, compared with that in the general population, was assessed with standardized incidence ratios,9 whereby observed cases were compared with the number expected by applying age-specific and sex-specific incidence rates for WM in the white cohort from the Iowa Surveillance, Epidemiology, and End Results Program.10 The age-specific and sex-specific incidence rates of amyloidosis were based on data from Olmsted County, Minnesota.11
Results
Of the 452 patients with WM diagnosed at Mayo Clinic between January 1, 1974, and December 31, 1995, 87 patients (19%) were considered potential candidates for this study. Thirty-nine patients were excluded because of: (1) lack of a bone marrow examination within 30 days of diagnosis in 27 patients; (2) no information on the results of the bone marrow examination in 7 patients; and (3) biclonal gammopathy (monoclonal IgM plus IgG or IgA) in 5 patients. The remaining 365 patients were excluded because they had received therapy. Forty-eight (11%) of the 452 patients with WM were considered eligible for this study.
Clinical characteristics at diagnosis
The median age at diagnosis of these 48 patients was 63 years (range, 39-87 years). Only 1 patient (2%) was younger than 40 years of age, whereas 7 (15%) were younger than 50 years. Thirty-two patients (67%) were male, whereas 16 (33%) were female (Table 1).
Clinical and laboratory features among 48 patients with SWM
| . | Progression to WM (N = 34) . | Nonprogression (N = 12) . | Total (N = 48) . | Comments . |
|---|---|---|---|---|
| Male (%) | 65 | 83 | ||
| Age at diagnosis, y | ||||
| Median | 62 | 65 | 63 | Only 1 person < 40 y |
| Range | 39-87 | |||
| Liver palpable, N* | 4 | 1 | ||
| Spleen palpable, N* | 1 | 1 | ||
| Lymphadenopathy, N* | 4 | 0 | ||
| Hemoglobin, g/dL | ||||
| Median | 11.4 | 12.8 | 11.8 | < 10 g/dL in 8% |
| Range | 8.7-14.1 | 8.7-15.3 | 8.7-15.3 | < 12 g/dL in 54% |
| Serum monoclonal protein, g/dL | ||||
| Median | 3.4 | 3.0 | 3.3 | < 3 g dL in 25% |
| Range | 1.5-5.2 | 1.8-4.0 | 1.5-5.2 | ≥ 4 g/dL in 21% |
| Serum creatinine, mg/dL | ||||
| Median | 1.1 | 1.1 | 1.1 | > 2 mg/dL in 2% (missing in 2) |
| Range | .6-2.1 | .7-1.4 | .6-2.1 | |
| β2-microglobulin, μg/mL | ≤ 1.8 μg/mL in 4 | |||
| Median | 2.1 | 2.2 | 2.14 | ≥ 3 μg/mL in 3 (missing in 26) |
| Range | 1.6-4.0 | 1.5-3.2 | 1.5-4.0 | |
| Urine monoclonal protein, g/24 hours | < 0.1 g/24 h in 70% | |||
| Median | .1 | .05 | > 0.2 g/24 h in 17% | |
| Range | Not measurable to 1.4 | Not measurable to 0.3 | Not measurable to 1.4 | > 1.0 g/24 h in 3% (missing in 18) |
| Bone marrow lymphoplasmacytic cells, % | ||||
| Median | 40 | 15 | 30 | ≥ 10% in 94% |
| Range | 8-80 | 3-30 | 3-80 | ≥ 50% in 27% |
| . | Progression to WM (N = 34) . | Nonprogression (N = 12) . | Total (N = 48) . | Comments . |
|---|---|---|---|---|
| Male (%) | 65 | 83 | ||
| Age at diagnosis, y | ||||
| Median | 62 | 65 | 63 | Only 1 person < 40 y |
| Range | 39-87 | |||
| Liver palpable, N* | 4 | 1 | ||
| Spleen palpable, N* | 1 | 1 | ||
| Lymphadenopathy, N* | 4 | 0 | ||
| Hemoglobin, g/dL | ||||
| Median | 11.4 | 12.8 | 11.8 | < 10 g/dL in 8% |
| Range | 8.7-14.1 | 8.7-15.3 | 8.7-15.3 | < 12 g/dL in 54% |
| Serum monoclonal protein, g/dL | ||||
| Median | 3.4 | 3.0 | 3.3 | < 3 g dL in 25% |
| Range | 1.5-5.2 | 1.8-4.0 | 1.5-5.2 | ≥ 4 g/dL in 21% |
| Serum creatinine, mg/dL | ||||
| Median | 1.1 | 1.1 | 1.1 | > 2 mg/dL in 2% (missing in 2) |
| Range | .6-2.1 | .7-1.4 | .6-2.1 | |
| β2-microglobulin, μg/mL | ≤ 1.8 μg/mL in 4 | |||
| Median | 2.1 | 2.2 | 2.14 | ≥ 3 μg/mL in 3 (missing in 26) |
| Range | 1.6-4.0 | 1.5-3.2 | 1.5-4.0 | |
| Urine monoclonal protein, g/24 hours | < 0.1 g/24 h in 70% | |||
| Median | .1 | .05 | > 0.2 g/24 h in 17% | |
| Range | Not measurable to 1.4 | Not measurable to 0.3 | Not measurable to 1.4 | > 1.0 g/24 h in 3% (missing in 18) |
| Bone marrow lymphoplasmacytic cells, % | ||||
| Median | 40 | 15 | 30 | ≥ 10% in 94% |
| Range | 8-80 | 3-30 | 3-80 | ≥ 50% in 27% |
Information not available on 7 patients.
At diagnosis, the liver was palpable in 5 patients (12%), whereas the spleen was palpable in 2 patients (5%). Only 1 patient (2%) had enlargement of both liver and spleen. Lymphadenopathy was noted in 4 patients (10%).
The initial hemoglobin level ranged from 8.7 to 15.3 g/dL (median, 11.8 g/dL; Table 1). The anemia in all 4 patients with an initial hemoglobin value less than 10 g/dL was the result of other causes (myelodysplastic syndrome, bronchopleural fistula with empyema, Barrett esophagus with bleeding, and chronic renal failure unrelated to WM). All 4 patients with a hemoglobin < 10 g/dL had < 50% lymphoplasmacytic infiltration of the bone marrow. The initial median leukocyte level was 5.8 × 109/L, and the median platelet value was 278 × 109. No patient had < 3.2 × 109 leukocytes or < 100 × 109 platelets.
The median creatinine value was 1.1 mg/dL. Only one patient (2%) had a value greater than 2 mg/dL, and that was attributed to aortic valve disease and congestive heart failure unrelated to the SWM. The serum monoclonal (M) protein level at the time of the diagnosis ranged from 1.5 g/dL to 5.2 g/dL (median, 3.3 g/dL). Twelve (25%) were < 3 g/dL and 10 (21%) were ≥ 4 g/dL. IgM κ was present in 75%, IgM λ in 21%, and 4% were biclonal (both had an IgM component). Levels of uninvolved (normal, polyclonal, or background) immunoglobulins were reduced in 18 (47%) of 38 patients whose immunoglobulin levels were determined quantitatively. Ten (26%) had a reduction of one uninvolved immunoglobulin, and 8 (21%) had a reduction of both IgG and IgA immunoglobulins (eg, reduced IgA and IgG in a patient with a monoclonal IgM). There was no reduction in uninvolved immunoglobulins in 20 patients (53%).
The β2-microglobulin value was available in 22 patients and ranged from 1.5 μg/mL to 4.0 μg/mL (median, 2.1 μg/mL; normal values, 0.7-1.8 mg/mL). A total of 82% were greater than 1.8 μg/mL.
The amount of urine M protein ranged from not measurable to 1.4 g/24 hours (median, 0.05 g/24 hours) in the 42 patients (88%) who were tested within 30 days of the diagnosis. Only 5 patients had a urine M protein more than 200 mg/24 hours (17%). Immunofixation of the urine revealed κ in 25 (59.5%), λ in 5 (12%), and negative in the remaining 12 patients (28.5%) tested. Only 1 patient had > 100 mg of urine albumin (384 mg/24 hours). Serum albumin ranged from 2.5 g/dL to 4.3 g/dL (median, 3.6 g/dL). Five patients (10%) had a serum albumin level < 3 g/dL.
Bone marrow aspirate and biopsy specimens were examined in all patients. Lymphoplasmacytic infiltration ranged from 3% to 80% (median, 30%). Only 3 patients (6%) had < 10% infiltration, whereas 13 (27%) had ≥ 50% infiltration (Table 1).
Clinical course and disease progression
During 285 cumulative person-years of follow-up (median, 15.4 years; range, 0.4-24.6 years), 34 (71%) progressed to WM requiring chemotherapy, 1 to AL amyloidosis, and 1 to lymphoma (total, 75%). On the basis of the Surveillance, Epidemiology, and End Results data, one would have expected 0.004 cases of WM; thus, the relative risk of progression was increased 8034-fold (95% CI, 5563-11 227) for the whole cohort.
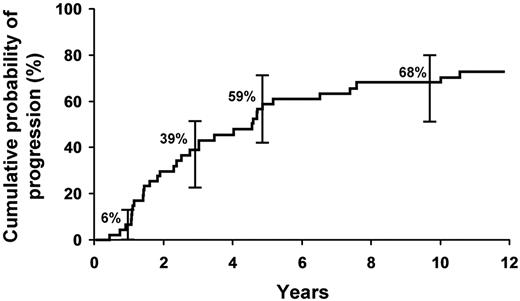
The cumulative probability of progression to symptomatic WM requiring therapy, amyloidosis, or lymphoma was 6% at 1 year, 39% at 3 years, 59% at 5 years, and 68% at 10 years (Figure 1). The cumulative probability of progression was 12% per year for the first 5 years and then 2% per year for the next 5 years for the entire group.
Cumulative probability of progression to symptomatic WM requiring therapy, amyloidosis, or lymphoma in the cohort of 48 patients with SWM.
Cumulative probability of progression to symptomatic WM requiring therapy, amyloidosis, or lymphoma in the cohort of 48 patients with SWM.
Risk factors for progression
We evaluated baseline factors with respect to progression to symptomatic WM. The risk factors studied included sex, hemoglobin level, size of serum M protein, type of serum light chain, free light chain (FLC) ratio, and difference between involved and uninvolved FLC, reduction of uninvolved immunoglobulins, presence of urinary monoclonal light chains, serum albumin level, and the percentage of lymphoplasmacytic cells in the bone marrow.
On the basis of univariate analysis, the percentage of bone marrow lymphoplasmacytic cells, size of the serum electrophoretic spike, hemoglobin value, and presence of IgA reduction were identified as significant factors for progression at a P value < .05.
On multivariate analysis, the percentage of lymphoplasmacytic cells in the bone marrow was most important. The size of the serum M spike and hemoglobin value each contributed significantly to a model already containing the percentage of lymphoplasmacytic cells in the bone marrow as significant risk factors for progression (Table 2).
Risk factors for progression to treatment among 48 patients with SWM
| Multivariate model . | Hazard ratio . | P . |
|---|---|---|
| Bone marrow lymphoplasmacytic cells | 1.31 (1.1-1.5) | < .001 |
| Hemoglobin value | 0.7 (0.5-0.9) | .002 |
| Serum M protein value | 2.1 (1.3-3.5) | .003 |
| IgA reduction | 2.4 (1.1-5.4) | .036 |
| Multivariate model . | Hazard ratio . | P . |
|---|---|---|
| Bone marrow lymphoplasmacytic cells | 1.31 (1.1-1.5) | < .001 |
| Hemoglobin value | 0.7 (0.5-0.9) | .002 |
| Serum M protein value | 2.1 (1.3-3.5) | .003 |
| IgA reduction | 2.4 (1.1-5.4) | .036 |
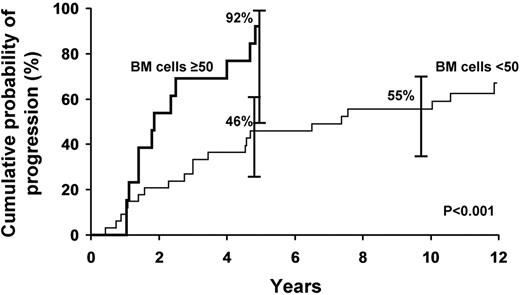
The progression rate during the first 5 years was 92% for the 13 patients who had 50% or more lymphoplasmacytic cells in the bone marrow at diagnosis and 46% for those with bone marrow infiltration of less than 50% (P = .001; Figure 2). The risk of progression at 5 years was 61% in those with an M protein ≥ 3 g/dL and 10% or more bone marrow lymphoplasmacytic involvement, whereas the risk of progression at 5 years was 49% with those with an M-spike < 3 g/dL and a bone marrow lymphoplasmacytic level ≥ 10%. The difference between these latter 2 groups was not statistically significant (P = .265; Figure 3).
Probability of progression to symptomatic WM requiring therapy on the basis of lymphoplasmacytic bone marrow infiltration.
Probability of progression to symptomatic WM requiring therapy on the basis of lymphoplasmacytic bone marrow infiltration.
Probability of progression to symptomatic WM with M-spike ≥ 3 g/dL and bone marrow cells ≥ 10% and M-spike < 3 g/dL and bone marrow cells ≥ 10%.
Probability of progression to symptomatic WM with M-spike ≥ 3 g/dL and bone marrow cells ≥ 10% and M-spike < 3 g/dL and bone marrow cells ≥ 10%.
In the 12 patients who did not progress to active disease, the baseline median level of lymphoplasmacytic cells in the bone marrow was 15%, whereas the median size of the serum monoclonal protein spike was 3.0 g/dL and uninvolved immunoglobulins were reduced in 3 of 10 who were measured compared with 40% infiltration and 3.3 g/dL protein spike, respectively, in the 36 patients who progressed.
Survival
During the 285 cumulative person-years of follow-up to progression, 75% of the 48 patients with SWM died. All were followed up until December 31, 2010, except for one patient from Europe who was lost to follow-up, and that occurred 15 years after the diagnosis of symptomatic WM. The overall survival for the 48 SWM patients was 83% at 5 years and 50% at 9.6 years (Figure 4). Seven patients died of cardiovascular disease or other unrelated disorders before developing WM or a related disorder. The median survival after progression to symptomatic WM was 5.1 years (Figure 5). Only 5 SWM patients are alive and still at risk for progression; they have been followed now for 14 to 24.6 years.
Discussion
In 2003, a consensus panel proposed a definition of WM as having an IgM M protein of any concentration, presence of lymphoplasmacytic bone marrow infiltration (no minimum level specified), and no features of other lymphoproliferative disorders.3 The criteria we use differ2 and are based on subsequent studies.12-14 We consider asymptomatic patients with an IgM M protein < 3 g/dL and lymphoplasmacytic infiltration less than 10% as IgM MGUS, and not WM.2 Because these patients have a survival similar to the general population and require no therapy, they should not be erroneously labeled as having a malignancy. In this study, we define the natural history of SWM, which like IgM MGUS has an indolent clinical course and must be differentiated from WM.13,14 The diagnosis of SWM requires a serum monoclonal protein level of > 3 g/dL and/or 10% or more lymphoplasmacytic cells in the bone marrow and absence of symptomatic anemia, constitutional symptoms, hyperviscosity, symptomatic lymphadenopathy, or hepatosplenomegaly that can be attributed to the underlying lymphoproliferative disorder.2 In addition to the M spike and bone marrow infiltration, patients may have mild anemia, reduction of uninvolved serum immunoglobulins, modest reduction of serum albumin levels, or elevation of β2-microglobulin. Furthermore, some of these patients may have modest hepatosplenomegaly or lymphadenopathy. Almost 20% of our patients designated as WM actually had SWM.
SWM resembles IgM MGUS in that it is asymptomatic, but it is far more likely to progress to symptomatic WM, amyloidosis, or lymphoma at 10 years (65% vs 18% for IgM MGUS).12 In a report of 213 patients with IgM MGUS, 29 progressed to non-Hodgkin lymphoma (n = 17), WM (n = 6), chronic lymphocytic leukemia (n = 3), or AL amyloidosis (n = 3) with relative risks of 15, 262, 6, and 16, respectively. The cumulative probability of progression to 1 of these disorders was 10% at 5 years, 18% at 10 years, and 24% at 15 years. The overall average risk for progression was approximately 1.5% per year, and the major risk factor for progression was the size of the serum M protein at the time of recognition of IgM MGUS.12 In contrast, we show in this study that SWM has a greater than 10-fold higher risk of progression compared with IgM MGUS, necessitating distinction of this condition from IgM MGUS because of the prognostic implications, and the need for closer follow-up. In this regard, SWM resembles smoldering multiple myeloma (SMM), which is distinguished from MGUS clinically based on a higher risk of progression to myeloma or related malignancy.15
Just as it is important to distinguish patients with SWM from IgM MGUS, patients with SWM must also be differentiated from patients with symptomatic WM who have clear evidence of end-organ damage requiring therapy. As shown in this report, patients with SWM are asymptomatic and may remain so for many years. More importantly, our study shows that the overall survival of SWM is only modestly decreased compared with an age-matched general population. Indeed, a study by Baldini et al demonstrated that the overall survival of SWM patients is similar to that of the general population, making it more important that these patients be recognized clinically and avoid the risks of chemotherapy.13 Of interest, Gobbi et al previously reported that the overall survival was similar to that of the general population,14 attributable perhaps to closer medical surveillance resulting in better care of hypertension, diabetes, and other common disorders. It is therefore in the patient's best interest to recognize this entity so that these patients are not subjected to the risks of chemotherapy until evidence of progression to symptomatic WM. There are no data that early therapy of SWM prolongs survival compared with therapy at the time of symptoms. The median survival of WM patients in this cohort of patients with SWM is no different than that reported in the literature for WM during the time of this study. Thus, there is no reason to treat patients with SWM.
Progression of SWM is almost limited to the subsequent development of symptomatic WM, but an occasional patient may develop AL amyloidosis or lymphoma. Interestingly, progression is common during the first 5 years of follow-up in that nearly 60% of our patients with SWM progressed, but the progression rate decreased to approximately 2% annually for the next 5 years. This is similar to our finding in SMM.15 The most important risk factors in our study for progression of SWM were the degree of lymphoplasmacytic infiltration of the bone marrow, hemoglobin value, and size of the IgM monoclonal protein. It is of interest to note that 27% of our patients presented with ≥ 50% infiltration of the bone marrow at the time of recognition. It was surprising to find that neither the FLC ratio nor the difference between involved and uninvolved κ and λ values were risk factors for progression because an abnormal FLC ratio is a significant prognostic parameter for progression in MGUS,16 solitary plasmacytoma of bone,17 and SMM.18 Leleu reported that reduction of the involved FLC was a prognostic feature in response and progression of WM.19 Alexanian et al have suggested that disease progression of SWM is greater with lower baseline hemoglobin, elevated serum β2-microglobulin, and in patients with IgM M protein > 3.0 g/dL.20 Combinations of these 3 variables defined 3 risk groups with median times to progression of greater than 5 years, 2 years, and 0.5 years, respectively. Patients at high risk of progression should be considered for clinical trials.
In conclusion, we show that the risk of progression of SWM is highest in the first 5 years after diagnosis (∼ 12% per year) and then decreases to rates similar to IgM MGUS. Patients can sometimes be observed for years without therapy, and there are no randomized data that treatment of patients with minimal or no symptoms and low tumor burden results in prolongation of overall survival. Our results mirror findings by Greco et al who estimate a median survival in excess of 14 years in patients with SWM.21 Some patients with SWM who are at high risk for progression may be candidates for treatment on clinical trials. However, on the basis of our observations and the survival results reported by Baldini et al,13 we suggest that the standard of care for patients with SWM should be close follow-up every few months without therapy. The pertinent laboratory tests should be repeated 2 to 3 months after initial recognition of SWM to exclude early activity; if the results are stable, the studies should be repeated every 4 to 6 months. Patients at high risk of progression are candidates for clinical trials, but it is important to select only patients at high risk of progression and to use therapeutic agents with low toxicity, few side effects, and low cost.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the National Cancer Institute (grants CA62242, CA107476, and CA15083) and the JABBS Foundation and Predolin Foundation.
National Institutes of Health
Authorship
Contribution: R.A.K., T.M.T., L.J.M., and S.V.R. designed the study; R.A.K. analyzed the data and wrote the manuscript; J.T.B., D.R.L., and T.M.T. performed the statistical analyses; and S.V.R., A.D., S.K., and L.J.M. contributed to the analysis and provided critical review.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert A. Kyle, MD, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: kyle.robert@mayo.edu.