The cell lineage origin of interferon-producing killer dendritic cells (IKDCs) has been challenged since 2006 when the unique capacity of these cells to accumulate in tumors and mediate remarkable antitumor effects was first described.1-4 In this issue of Blood, Guimont-Desrochers et al redefine B220+ NK cells as a novel intermediate in NK-cell differentiation preceding the acquisition of CD27.5
IKDCs were originally defined as CD11cintB220+CD49b+MHC class II+ cells, endowed with an unexpected functional plasticity compared with classical NK cells. After in vivo exposure to imatinib mesylate and IL-2 or TLR9L, conditions of trans-presentation of IL-15Rα/IL-15, IKDCs lost MHC class II molecules and harbored potent TRAIL-dependent lytic activity leading to immunogenic cell death of their targets.6 In contrast, when licensed by tumor cells, IKDCs up-regulated MHC class II and CD86 molecules and acquired the capacity to cross-present exogenous antigens to naive CD8+ and CD4+ T cells in an MHC class I– and class II–dependent fashion, respectively.4,7 On TLR-9 activation in vitro, IKDCs down-regulated NKG2D/DAP10/DAP12 molecules as well as their killing potential and started producing IL-12p40.2 In vivo, MCMV infection triggered their migration to lymph nodes and their maturation into professional APCs able to cross-prime MCMV-specific CD8+ T cells.2 After lung viral infection with influenza virus, IKDCs were recruited to lungs and were endowed with antigen-presenting capacities.8 Most of these studies demonstrated the intrinsic APC functions of IKDCs using adoptive transfer of IKDCs in vivo (purified from resting spleens or cultured from bone marrow in the presence of growth factors) into hosts presenting genetic defects in the MHC class I4,7 or class II pathway7 or depleted from CD11chigh classical dendritic cells (DCs). In these reports, IKDCs drastically differed from B220− or MHC class II− NK cells that failed to present antigens to naive T cells.2,7
B220 is a lineage associated– rather than an activation marker in the NK-cell differentiation pathway and defines pre-mNK cells. Guimont-Desrochers et al adoptively transferred various NK-cell subsets and followed their proliferation and phenotype in immunocompetent versus Rag1-deficient recipients treated or not with activating stimuli (known to up-regulate B220 molecules in vitro). While IKDCs remained B220 at early time points without activation (and maintained this expression at later time points after activation), B220− NK cells (expressing or not CD27) failed to acquire B220. The follow-up study of B220+ NK cells revealed that this subset differentiated into maturing NK cells over time, losing B220, Ly108, and CD27 while acquiring CD11b and CD43 by day 25.
B220 is a lineage associated– rather than an activation marker in the NK-cell differentiation pathway and defines pre-mNK cells. Guimont-Desrochers et al adoptively transferred various NK-cell subsets and followed their proliferation and phenotype in immunocompetent versus Rag1-deficient recipients treated or not with activating stimuli (known to up-regulate B220 molecules in vitro). While IKDCs remained B220 at early time points without activation (and maintained this expression at later time points after activation), B220− NK cells (expressing or not CD27) failed to acquire B220. The follow-up study of B220+ NK cells revealed that this subset differentiated into maturing NK cells over time, losing B220, Ly108, and CD27 while acquiring CD11b and CD43 by day 25.
However, several arguments indicated that IKDCs may truly belong to the NK-cell lineage (as opposed to a bona fide DC origin), challenging the acronym “IKDC.” First, IKDCs were dependent on IL-15, IL-2Rγ chain, and Id2 but independent of Flt3L for their in vivo differentiation.6,9,10 Secondly, gene- and NK receptor–expression profiling suggested that IKDCs resemble mature NK cells.5 Finally, many IKDC-related markers (CD11c, B220, and MHC class II) can be shared by mature NK cells, at least on in vitro activation.9 Therefore, a consensual view emerged as to reconsidering IKDCs as B220+ NK cells endowed with unique tumoricidal and/or APC potential.
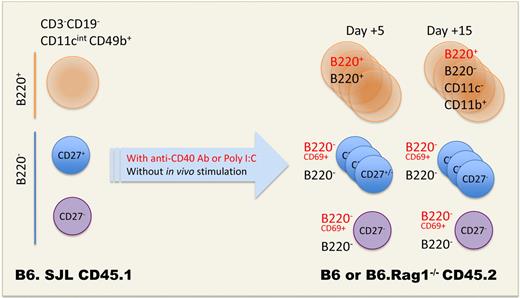
In this issue of Blood, Guimont-Desrochers et al report that B220+ NK cells are a novel intermediate in NK-cell differentiation preceding the acquisition of CD27.5 After adoptive transfer of CD45.1+B220+ NK cells (CD11cintCD122+) into C57BL/6 or B6.Rag1−/− CD45.2+ hosts, B220+ NK cells highly proliferated and progressively adopted a mature NK phenotype (B220−CD43+CD11bhigh). In contrast, B220− or CD27+ NK cells failed to acquire the so-called B220 activation marker even on in vivo stimulation with Poly I:C or anti-CD40 agonistic antibodies (see figure). The high incorporation rate of BrdU as well as a transcriptional profile highlighting genes associated with cell-cycle and microtubule organization, a large size, and high levels of the SLAM family member Ly108 are strong arguments supporting the immature phenotype of B220+ NK cells. Hence, the authors conclude that B220+ NK cells do not arise from the activation of mature NK cells and should rather be considered as immediate precursor of CD27+ immature NK cells.5 They propose to apostrophe IKDCs as “pre-mNK” for pre-mature NK cells that refer to CD3−CD19−CD49b+CD122+NKp46+ CD11cintB220+CD27+CD11bint cells.
Guimont-Desrochers et al discuss the important issue of what regulates the accumulation of pre-mNK cells in mice.5 In a previous work, they showed that genetic polymorphisms encoded within the distal region of mouse chromosome 7 (and related to hematopoietic factors) play a role in their proportions in that nonobese diabetic and B10.Br mice showed the lowest and highest proportion of pre-mNK (around 0.07% and 0.25% of splenocytes, respectively).11 Others reported the presence of CD27+ NK cells in some gene-targeted mouse models (Gata3, T-bet, Bcl11b), but more information is needed on which additional transcription factors (among Id2, PU1, Ets-1, Ikaros, TOX, E4BP4, MEF, Blimp-1, Eomes) are coordinated to give rise to B220+ NK cells. Of note, Welner et al pioneered the notion that such pre-mNK and mNK cells could arise from different bone marrow progenitors in that L-selectin+ lymphoid progenitors (LSPs) could electively give rise to pre-mNK cells while mature NK cells could differentiate from not only LSPs but also early and common lymphoid progenitors.10
The intriguing notion that emerges from this controversy is that in contrast to other lineages, NK cells may harbor distinct functions at distinct stages of their differentiation (TRAIL expression, secretion of TNFα or IFNγ, degranulation and cytotoxicity, APC function). We recently added some more complexity by showing that immature CD117/Kit+CD11b− NK cells accumulate during the progression of tumors secreting IL-18 and induce PDL-1/PD-1–dependent immunosuppressive functions.12 It is tempting to speculate that various local microenvironments and their peculiar growth factors may shape the maturation stage and hence, the function of NK cells residing in the periphery for optimal reactivity to pathogens, tumors, or allergens.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■