Abstract
Immune reconstitution inflammatory syndrome (IRIS) is a common and potentially serious complication occurring in HIV-infected patients being treated for tuberculosis (TB) using combined antiretroviral treatment. A role of adaptive immunity has been suggested in the onset of IRIS, whereas the role of natural killer (NK) cells has not yet been explored. The present study sought to examine the involvement of NK cells in the onset of IRIS in HIV-infected patients with TB and to identify predictive markers of IRIS. A total of 128 HIV-infected patients with TB from the Cambodian Early versus Late Introduction of Antiretroviral Drugs (CAMELIA) trial were enrolled in Cambodia. Thirty-seven of the 128 patients developed IRIS. At inclusion, patients had low CD4 cell counts (27 cells/mm3) and high plasma viral load (5.76 and 5.50 log/mL in IRIS and non-IRIS patients, respectively). At baseline, NK-cell degranulation capacity was significantly higher in IRIS patients than in non-IRIS patients (9.6% vs 6.38%, P < .005). At IRIS onset, degranulation capacity did not differ between patients, whereas activating receptor expression was lower in IRIS patients. Patients with degranulation levels > 10.84% had a higher risk of IRIS (P = .002 by log-rank test). Degranulation level at baseline was the most important IRIS predictor (hazard ratio = 4.41; 95% confidence interval, 1.60-12.16). We conclude that NK-degranulation levels identify higher IRIS risk in HIV-infected patients with TB.
Introduction
In 2009, approximately 33 million people were living with HIV infection worldwide, and 9.4 million persons were newly diagnosed with tuberculosis (TB).1 Of the 9.4 million, an estimated 1.0-1.2 million cases involved HIV infection and 0.38 million HIV-infected TB patients died.1 TB is the most common opportunistic infection and one of the most common causes of death in HIV-infected patients living in developing countries. The use of combined antiretroviral treatment (cART) during TB treatment improves survival by restoring immune functions.2 However, simultaneous treatment with antiretroviral drugs and anti-TB drugs can cause an excessive immune response known as the immune reconstitution inflammatory syndrome (IRIS).3 The general incidence of IRIS has been reported to be 19%-38%. TB-associated IRIS (TB-IRIS) is more frequent in areas where the prevalence of TB is high, such as Cambodia, 1 of the 22 World Health Organization–designated TB high-burden countries.1
Adaptive immunity has often been implicated in the pathogenesis of IRIS, whereas the role of natural killer (NK) cells has not yet been explored. It is known that NK cells influence the outcome of Mycobacterium tuberculosis infection4 and probably that of HIV-1 infection as well.
NK cells are granular lymphocytes5 with an important role in allograft rejection and killing of malignant and pathogen-infected cells. NK-cell activity is controlled by cytokines (mainly IL-12, IL-15, IL-18, and IFN-α) and by a complex repertoire of activating and inhibitory receptors.6 NK cells appear to play an important role in HIV infection.7,8 Significant alterations of NK-cell function and repertoire expression after HIV infection have been described previously.8 Stronger NK-cell activity and expression of natural cytotoxic receptors are associated with preservation of CD4 functions and with lower viral load.8 Furthermore, genetic associations between KIR haplotypes and slower progression to AIDS or even resistance to HIV infection are now well established.9-11 We have also characterized a subset of NK cells able to control HIV replication in dendritic cells.12
Although NK cells are capable of mounting a vigorous response to M tuberculosis, their precise role during TB is unknown. Granule-associated cytotoxic molecules from human NK cells are delivered to intracellular mycobacteria and have been shown to kill M tuberculosis.13 Human NK cells can lyse M tuberculosis–infected monocytes directly in vitro.14 This NK lysis seems to be mediated by NKG2D and NKp46, which bind to stress-induced ligand protein and vimentin, respectively.15 In vitro, human NK cells have been shown to induce specific CD8 T-cell responses by killing either infected macrophages16 or regulatory T cells17 and by enhancing cross-talk with dendritic cells in the presence of M tuberculosis,13 indicating that NK cells are important for the induction of adaptive immune responses to TB. Diagnosis of IRIS is difficult and no predictive tests are yet available. Although NK cells were studied in TB-IRIS,18 their role in TB-associated IRIS in HIV-infected patients has not yet been explored.
The aim of this study was to investigate the involvement of NK cells in the occurrence of TB-IRIS in HIV-infected patients with TB who started cART and to identify markers of innate immunity that could predict the occurrence of IRIS in this setting.
Methods
Patients
This study was linked to the Cambodian Early versus Late Introduction of Antiretroviral Drugs (CAMELIA) clinical trial (ANRS 1295-CIPRA KH 001-DAIDS-ES ID 10425), which demonstrated markedly improved survival when cART was started at 2 weeks versus 8 weeks after TB treatment initiation.19 From June 2006 to August 2008, 147 HIV-infected adults with TB enrolled in the CAMELIA trial in Cambodia gave their specific signed consent to enter this study. CAMELIA was a prospective, randomized, multicenter, open-label, 2-arm superiority trial conducted in Cambodia.19 All patients started TB treatment and then started cART either 2 or 8 weeks later.2,19 During this trial, IRIS was defined as a worsening or emergence of signs or symptoms of TB at any time after cART initiation during appropriate TB treatment to which the patient had initially responded. Each suspected case of IRIS was validated by a chart review conducted by at least 2 members of the clinical coordination team, who ensured that the findings could not be attributed to a newly acquired infection, clinical progression of drug-resistant TB, the expected clinical course of a previously recognized infection, or adverse effects of cART. Three control groups were also studied: HIV-infected patients naive of cART with no evidence of TB (HIV+/TB−, n = 49), HIV-seronegative TB patients (HIV−/TB+, n = 30) recruited in participating hospitals in Cambodia, and healthy subjects who were HIV-seronegative and had no evidence of TB (HIV−/TB−, n = 30), recruited from the Voluntary Confidential Counselling and Testing Center at the Pasteur Institute of Cambodia. HIV+/TB+ patients enrolled in the CAMELIA study provided blood samples after 2 weeks of TB treatment and at the time of IRIS diagnosis. Samples from HIV+/TB− patients were obtained before cART initiation; those from TB+/HIV− patients were obtained 2 weeks after TB treatment initiation. CD4 cell counts and HIV viral load were determined in a central laboratory (Pasteur Institute in Cambodia). This study was approved by the Cambodian National Ethics Committee for Human Research and the clinical ethics committee of the Pasteur Institute.
Phenotypic studies of NK cells
All phenotypic studies were done on fresh whole blood using a 4-color FACSCalibur II flow cytometer (BD Biosciences) and FlowJo Version 5.0 software (TreeStar). Lymphocytes were gated in the FSC/SSC dot plot and NK cells were identified as CD3− and CD56+ and/or CD16+ lymphocytes. NK-cell subsets were identified by coexpression of CD56 and CD16 on NK cells: BRIGHTNK cell (CD56++CD16−), INTCD56 (CD56+CD16−), DIMCD56 (CD56+CD16+), and POSCD16 (CD56−CD16+).
NK-cell receptors (NKp30, NKp44, NKp46, and NKp80); NK cell Ig-like receptors (KIR; CD158a, 158b, 158e, and 158i); CD160, CD161, NKG2A, NKG2C, NKG2D, DNAM, CD122, CD244, and the activation marker CD69 were detected with mAbs (see supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) from Beckman Coulter and R&D Systems.
Degranulation (CD107a expression) assay and intracellular cytokine staining
CD107a expression on NK cells and the ICS-based assay were used to measure degranulation and cytokine expression, as described previously.12 PBMCs were isolated from fresh whole blood and incubated overnight with the target cell line K562 at different NK cell/target ratios in the presence of conjugated CD107a (20 μg/mL; BD Biosciences). After 1 hour of incubation, brefeldin A (1 μg/mL; Sigma-Aldrich) and monensin (6 μg/mL; Sigma-Aldrich) were added. PBMCs incubated under the same conditions but without target cells were used to measure spontaneous degranulation and cytokine production. At the end of the incubation, NK cells were stained with Abs to CD56, CD16, CD3, and IFN-γ from Beckman Coulter and BD Biosciences. Total degranulation capacity and IFN-γ secretion was analyzed in total NK cells. Sample acquisition was done with a FACSCalibur flow cytometer (BD Biosciences) and analysis was done in FlowJo Version 9.4 software.
Statistical analysis
Baseline characteristics were compared across groups using the χ2 test for categorical variables and a t test or a nonparametric test depending on the sample size. Degranulation at baseline was first compared across the 5 groups using the Kruskal-Wallis test for multiple group comparisons. If the test showed a significant difference, 2-by-2 comparisons were conducted using the Student t test and applying Bonferroni correction. To identify the significant differences, we did not consider a significance level of P = .05, but of P = .05/10 = .005 (Bonferroni correction). NK-cell function at IRIS onset was compared by matching each HIV+/TB+ IRIS patient with 1 IRIS-free control for whom values were available within ± 2 weeks of the case values. The Wilcoxon nonparametric test for paired samples was used for this comparison.
The association between NK-cell activity before cART initiation and occurrence of IRIS was studied by constructing Kaplan-Meier curves. Each factor was subdivided into 4 categories, based on the 25th, 50th, and 75th percentiles, with these percentiles being estimated when both IRIS and non-IRIS patients were combined, and the curves were compared using the log-rank test. The effect of each factor was also quantified in a Cox model, the proportional hazard assumption was validated by the test based on Schoenfeld residuals.
Results
Patients
Of the 147 HIV+/TB+ patients from the CAMELIA trial initially enrolled in this NK-cell study, 19 were excluded from the final analysis for the following reasons: 10 patients had no follow-up on cART, 5 had suspected TB-IRIS that was not confirmed after review, 2 had TB paradoxical reactions before cART initiation, and 2 patients initiated cART before the scheduled date. Of the remaining 128 patients, 37 had confirmed TB-IRIS. Of the 63 HIV+/TB− controls, 14 were excluded for the following reasons: 9 had no follow-up on cART and 5 were not ART naive and/or had ARV-resistance mutations at enrollment.
At baseline, HIV+/TB+ patients (IRIS and non-IRIS) had similar demographic and clinical characteristics, including sex, age, body mass index, CD4+ and NK cell counts, and HIV RNA levels (Table 1). Their baseline characteristics were very similar to those of the patients enrolled in the CAMELIA trial (supplemental Table 1), so patients included in the present substudy are representative of the CAMELIA study population. In particular, the proportions of patients with disseminated TB were not different between the CAMELIA and the present study. The CD4-cell count was significantly lower in these HIV+/TB+ patients than in HIV+/TB− patients (both P < .001) and the viral load was higher in HIV+/TB+ IRIS patients than in HIV+/TB− patients (P = .004). The other immunologic parameters, including NK-cell counts, were similar.
Demographic and clinical characteristics
| . | HIV+/TB+ IRIS (n = 37) . | HIV+/TB+ non-IRIS (n = 91) . | HIV+/TB− (n = 49) . |
|---|---|---|---|
| Sex | |||
| Male | 23 (62.2%) | 57 (62.6%) | |
| Female | 14 (37.8%) | 34 (37.4%) | |
| Median age, y (IRQ) | 33 (28-38) | 36 (31-44) | |
| CD4, cells/mm3 | |||
| n (%) | 37 (100.0) | 91 (100.0) | 34 (69.4) |
| Median (IQR) | 27 (9-47) | 27 (10-68) | 85 (29-165)* |
| Viral load, log copies/mL | |||
| n (%) | 37 (100.0) | 91 (100.0) | 34 (65.4) |
| Median (IQR) | 5.76 (5.41-5.99) | 5.50 (5.10-5.95) | 5.40 (4.77-5.71)† |
| BMI, kg/m2 | |||
| Median (IQR) | 16.0 (15.4-18.4) | 16.8 (15.6-19.0) | |
| Identification | |||
| TB | 33 (89.2) | 62 (68.1) | |
| Atypical myco | 0 | 2 (2.2) | |
| Unknown | 4 (10.8) | 27 (29.7) | |
| TB disseminated | |||
| Yes | 16 (43.2) | 16 (17.6)‡ | |
| Total NK cells, % | |||
| n (%) | 32 (86.5) | 78 (85.7) | 49 (100.0) |
| Median (IQR) | 18.0 (10.0-28.3) | 20.4 (11.4-25.4) | 17.5 (12.5-22.5) |
| . | HIV+/TB+ IRIS (n = 37) . | HIV+/TB+ non-IRIS (n = 91) . | HIV+/TB− (n = 49) . |
|---|---|---|---|
| Sex | |||
| Male | 23 (62.2%) | 57 (62.6%) | |
| Female | 14 (37.8%) | 34 (37.4%) | |
| Median age, y (IRQ) | 33 (28-38) | 36 (31-44) | |
| CD4, cells/mm3 | |||
| n (%) | 37 (100.0) | 91 (100.0) | 34 (69.4) |
| Median (IQR) | 27 (9-47) | 27 (10-68) | 85 (29-165)* |
| Viral load, log copies/mL | |||
| n (%) | 37 (100.0) | 91 (100.0) | 34 (65.4) |
| Median (IQR) | 5.76 (5.41-5.99) | 5.50 (5.10-5.95) | 5.40 (4.77-5.71)† |
| BMI, kg/m2 | |||
| Median (IQR) | 16.0 (15.4-18.4) | 16.8 (15.6-19.0) | |
| Identification | |||
| TB | 33 (89.2) | 62 (68.1) | |
| Atypical myco | 0 | 2 (2.2) | |
| Unknown | 4 (10.8) | 27 (29.7) | |
| TB disseminated | |||
| Yes | 16 (43.2) | 16 (17.6)‡ | |
| Total NK cells, % | |||
| n (%) | 32 (86.5) | 78 (85.7) | 49 (100.0) |
| Median (IQR) | 18.0 (10.0-28.3) | 20.4 (11.4-25.4) | 17.5 (12.5-22.5) |
IQR indicates interquartile range.
P < .001 when comparing HIV+/TB+ IRIS and HIV+/TB+ non-IRIS with HIV+/TB−.
P = .004 when comparing HIV+/TB+ IRIS with HIV+/TB−.
P = .002 when comparing HIV+/TB+ IRIS with HIV+/TB+ non-IRIS.
NK subsets in peripheral blood before cART and at IRIS onset.
The distribution of NK subsets was altered in the HIV-infected patients (HIV+/TB+ IRIS, HIV+/TB+ non-IRIS and HIV+/TB−; supplemental Table 2) compared with HIV− controls. We observed, for the first time, an increase in the INTCD56 (CD56+CD16-) subset in HIV-infected patients compared with HIV−/TB+ and HIV−/TB− patients. We also confirmed some other modifications, such as the increase in the POSCD16 subset and a very significant decrease in the DIMCD56 population.20 At IRIS onset, there was no significant difference in NK-cell subsets between HIV+/TB+ patients with and without IRIS (data not shown).
Activation and receptor expression on NK cells from IRIS and non-IRIS patients
Before cART initiation, CD69 expression was higher on NK cells from HIV+/TB+ patients than in the controls (data not shown). In contrast, we found no difference in NK-cell receptor expression between HIV+/TB+ patients with and without IRIS (data not shown). Lower expression of natural cytotoxic receptors (NKp30, NKp44, and NKp46) was found in HIV-1–infected patients regardless of their TB and IRIS status, than in HIV−/TB− patients (data not shown), in agreement with previous reports.8
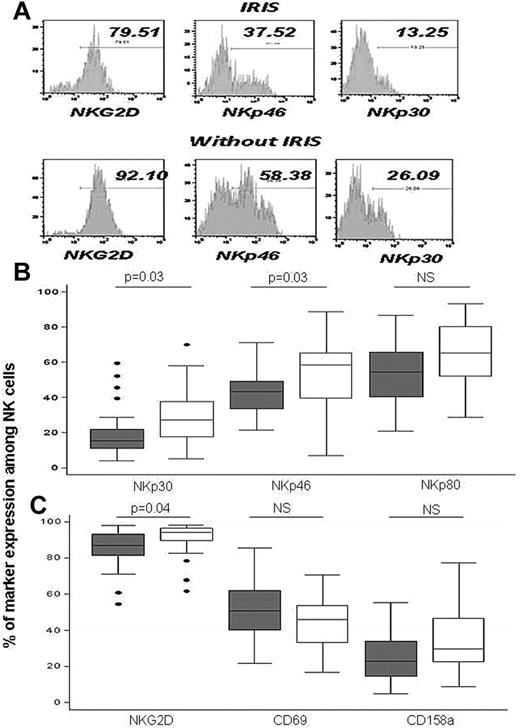
At IRIS onset, although the CD69 NK-cell activation marker was not different in HIV+/TB+ patients (IRIS and non-IRIS), the expression of NKp30, NKp46, and NKG2D (activating receptors) was significantly lower in IRIS patients, suggesting an inhibitory phenotype (Figure 1).
Expression of NK cell–activating receptors at IRIS onset. Typical flow cytometry results are show in panel A and overall results in panels B and C. Results are medians (75th-25th percentiles). Outside values are indicated by points. Significant P values are indicated. Gray boxes and white boxes indicate IRIS and non-IRIS patients, respectively.
Expression of NK cell–activating receptors at IRIS onset. Typical flow cytometry results are show in panel A and overall results in panels B and C. Results are medians (75th-25th percentiles). Outside values are indicated by points. Significant P values are indicated. Gray boxes and white boxes indicate IRIS and non-IRIS patients, respectively.
Degranulation and IFN-γ secretion before cART and at IRIS onset
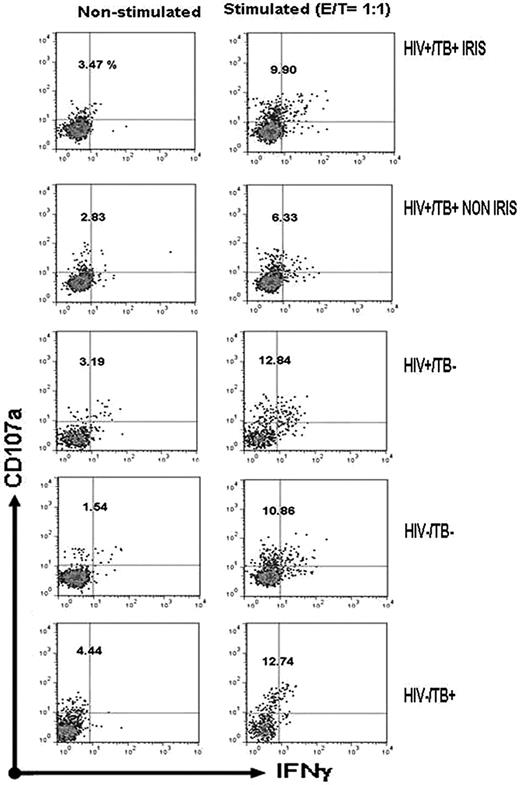
NK-cell degranulation and IFN-γ production before cART initiation was analyzed. Typical cytometric results are shown in Figure 2 and the overall results are shown in Figure 3 and Table 2. A significantly higher degranulation capacity after target-cell stimulation was observed in HIV+/TB+ IRIS patients compared with HIV+/TB+ non-IRIS patients in terms of both total CD107a expression (CD107a+IFN-γ+/−; P < .005; Figure 3A) and CD107a+ NK cells without IFN-γ secretion (Figure 3B; P < .005), whereas IFN-γ production was not different between these 2 groups (Figure 3C). The non-IRIS patients also had lower degranulation than all control groups (Figure 3A). A trend toward higher spontaneous degranulation of NK cells (P = .04) was also observed in IRIS patients, suggesting NK-cell activation in vivo (Table 2). Degranulation capacity was not different between HIV+/TB+ IRIS patients and HIV−/TB+ or HIV−/TB− controls, but was lower than in HIV+/TB− patients. When the total degranulation level between patients with and without disseminated TB was compared, we did not find evidence of a difference (supplemental Table 3). We also looked for a correlation between total degranulation capacity of NK cells at baseline and other parameters (CD4 and viral load) and no significant correlation was found (data not shown).
Flow cytometric analysis of NK-degranulation capacity. A typical pattern of CD107a expression and IFN-γ production is shown for unstimulated and K562 cell–stimulated PBMCs from the indicated groups. Dot plots are gated on NK cells. The percentage of total CD107a expression on NK cells is indicated.
Flow cytometric analysis of NK-degranulation capacity. A typical pattern of CD107a expression and IFN-γ production is shown for unstimulated and K562 cell–stimulated PBMCs from the indicated groups. Dot plots are gated on NK cells. The percentage of total CD107a expression on NK cells is indicated.
Degranulation capacity and IFN-γ production by target-stimulated NK cells before cART initiation. Total degranulation (total CD107a+ expression [A]), degranulation without IFN-γ production (CD107a+IFN-γ− [B]), and total IFN-γ production (IFN-γ+ [C]) are compared across the different groups. For HIV-infected patients, the results are those obtained before cART. Results are medians (75th-25th percentiles). Outside values are indicated by points. Significant P values are indicated.
Degranulation capacity and IFN-γ production by target-stimulated NK cells before cART initiation. Total degranulation (total CD107a+ expression [A]), degranulation without IFN-γ production (CD107a+IFN-γ− [B]), and total IFN-γ production (IFN-γ+ [C]) are compared across the different groups. For HIV-infected patients, the results are those obtained before cART. Results are medians (75th-25th percentiles). Outside values are indicated by points. Significant P values are indicated.
NK degranulation in nonstimulated conditions before cART in TB− IRIS patients
| . | HIV+/TB+ IRIS (n = 32)(1) . | HIV+/TB+ Non-IRIS (n = 67)(2) . | HIV+/TB− (n = 49) (3) . | HIV−/TB− (n = 30) (4) . | HIV−/TB+ (n = 28) (5) . | P < .005 . |
|---|---|---|---|---|---|---|
| Total CD107a | (1) vs (4) | |||||
| Median | 3.53 | 2.84 | 3.12 | 1.51 | 4.44 | (2) vs (4) |
| IQR | 1.57-6.10 | 1.55-3.99 | 2.07-4.18 | 1.00-2.83 | 2.85-7.47 | (2) vs (5) |
| CD107a+IFNγ− | (1) vs (3) | |||||
| Median | 3.06 | 2.31 | 1.67 | 1.03 | 3.77 | (1) vs (4) |
| IQR | 1.16-5.01 | 1.28-3.46 | 1.10-2.35 | 0.73-2.12 | 2.30-6.19 | (2) vs (4) |
| (2) vs (5) | ||||||
| CD107a+IFNγ+ | (1) vs (3) | |||||
| Median | 0.46 | 0.35 | 1.24 | 0.31 | 0.56 | (2) vs (3) |
| IQR | 0.28-0.83 | 0.20-0.66 | 0.72-1.74 | 0.24-0.89 | 0.36-1.11 | |
| CD107a−IFNγ+ | (1) vs (3) | |||||
| Median | 0.57 | 0.50 | 1.33 | 0.47 | 0.46 | (2) vs (3) |
| IQR | 0.27-1.16 | 0.29-0.77 | 0.70-2.23 | 0.31-0.69 | 0.14-0.88 | |
| Total IFNγ | (1) vs (3) | |||||
| Median | 1.18 | 0.92 | 2.76 | 0.88 | 1.24 | (2) vs (3) |
| IQR | 0.72-1.88 | 0.59-1.45 | 1.56-3.58 | 0.54-1.44 | 0.59-1.94 |
| . | HIV+/TB+ IRIS (n = 32)(1) . | HIV+/TB+ Non-IRIS (n = 67)(2) . | HIV+/TB− (n = 49) (3) . | HIV−/TB− (n = 30) (4) . | HIV−/TB+ (n = 28) (5) . | P < .005 . |
|---|---|---|---|---|---|---|
| Total CD107a | (1) vs (4) | |||||
| Median | 3.53 | 2.84 | 3.12 | 1.51 | 4.44 | (2) vs (4) |
| IQR | 1.57-6.10 | 1.55-3.99 | 2.07-4.18 | 1.00-2.83 | 2.85-7.47 | (2) vs (5) |
| CD107a+IFNγ− | (1) vs (3) | |||||
| Median | 3.06 | 2.31 | 1.67 | 1.03 | 3.77 | (1) vs (4) |
| IQR | 1.16-5.01 | 1.28-3.46 | 1.10-2.35 | 0.73-2.12 | 2.30-6.19 | (2) vs (4) |
| (2) vs (5) | ||||||
| CD107a+IFNγ+ | (1) vs (3) | |||||
| Median | 0.46 | 0.35 | 1.24 | 0.31 | 0.56 | (2) vs (3) |
| IQR | 0.28-0.83 | 0.20-0.66 | 0.72-1.74 | 0.24-0.89 | 0.36-1.11 | |
| CD107a−IFNγ+ | (1) vs (3) | |||||
| Median | 0.57 | 0.50 | 1.33 | 0.47 | 0.46 | (2) vs (3) |
| IQR | 0.27-1.16 | 0.29-0.77 | 0.70-2.23 | 0.31-0.69 | 0.14-0.88 | |
| Total IFNγ | (1) vs (3) | |||||
| Median | 1.18 | 0.92 | 2.76 | 0.88 | 1.24 | (2) vs (3) |
| IQR | 0.72-1.88 | 0.59-1.45 | 1.56-3.58 | 0.54-1.44 | 0.59-1.94 |
For all degranulation markers, the P value obtained from a Kruskal-Wallis test to compare the 5 groups was < .001; this allowed the 2-by-2 comparisons provided in this column. Only significant P values are indicated. Significantly different values are indicated in bold.
IQR indicates interquartile range.
At the time of IRIS, degranulation capacity increased in stimulated NK cells from HIV+/TB+ patients without IRIS (supplemental Table 4) and there was no difference between HIV+/TB+ IRIS patients and matched HIV+/TB+ patients without IRIS under either stimulated or nonstimulated conditions (supplemental Table 4).
Total stimulated NK-cell degranulation at baseline correlates with the risk of TB-IRIS
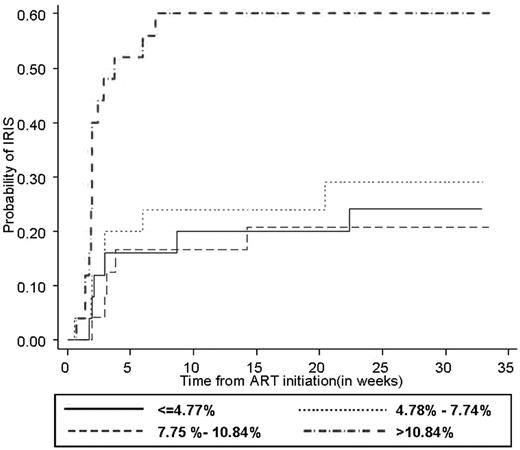
Kaplan-Meier estimates showed that the risk of IRIS was influenced by the level of stimulated NK-cell degranulation at baseline (P = .002 by log-rank test). Moreover, in patients with total NK degranulation > 10.84%, all IRIS occurred within 8 weeks from cART initiation (median = 14 days) after starting cART, indicating that higher NK-cell activity may be associated with early-onset IRIS. Total NK degranulation at baseline was the most important determinant of the risk of IRIS, which was 4-fold higher in patients with total NK degranulation > 10.84% (hazard ratio [HR] = 4.41; 95% confidence interval [95% CI], 1.60-12.16 for CD107a+ NK cells). Based on CD107a+IFNγ− NK cells, the risk of TB-IRIS was nearly 3 times higher in patients with NK degranulation > 7.88 (HR = 2.80; 95% CI, 1.06-7.37). In univariate analysis, age and disseminated TB were also significantly associated with the risk of IRIS (data not shown). When these 2 factors and total NK degranulation were entered in a multivariate Cox model, they all remained significantly associated with the risk of IRIS. After adjustment for age and disseminated TB, patients with total NK degranulation > 10.84 had more than a 4-fold increase in risk of IRIS (adjusted HR = 4.30; 95% CI, 1.60-11.57; Figure 4).
Probability of IRIS onset according to total NK-cell degranulation capacity before cART initiation. The association between total NK-cell activity before cART initiation and the occurrence of TB-IRIS is shown using Kaplan-Meier curves. Each factor was subdivided into 4 categories based on the 25th, 50th, and 75th percentiles. Plain line ≤ 1st quartile; dashed line, 1st quartile to median; dotted line, median to 3rd quartile; and dashed-dotted line > 3rd quartile (P = .002 by log-rank test).
Probability of IRIS onset according to total NK-cell degranulation capacity before cART initiation. The association between total NK-cell activity before cART initiation and the occurrence of TB-IRIS is shown using Kaplan-Meier curves. Each factor was subdivided into 4 categories based on the 25th, 50th, and 75th percentiles. Plain line ≤ 1st quartile; dashed line, 1st quartile to median; dotted line, median to 3rd quartile; and dashed-dotted line > 3rd quartile (P = .002 by log-rank test).
Discussion
We examined the potential role of NK cells in the occurrence of IRIS in HIV+/TB+ patients participating in a randomized clinical trial. We found that HIV+/TB+ patients who developed IRIS after cART initiation exhibited phenotypic and functional differences in their NK-cell compartments relative to their IRIS-free counterparts. Patients who developed IRIS had significantly higher levels of NK-cell degranulation before cART initiation, indicating that NK-cell degranulation might serve as a predictive marker of IRIS. At the time of IRIS, degranulation activity was similar in the 2 groups, but differences were noted in the expression of NK-cell receptors, and especially activating receptors. A role of NK cells in unmasking TB-related IRIS in HIV-infected subjects has been also suggested in a recent study.18
To understand the role of NK cells in cART-induced immune reconstitution and in the onset of TB-IRIS, both NK cytotoxic activity and the interaction of these cells with other immune components against a wide range of opportunistic pathogens including TB must be considered. NK cells engage in cross-talk with other immune cells, including CD4 T cells, to promote adaptive immune responses.16,21 Increased CD4 T-cell activation before cART introduction has been reported in HIV+/TB+ patients,22 and it has been suggested that this activation is driven by specific Ag responses due to a higher Ag load or an imbalance in effector responses.22 The higher activity of NK cells found in IRIS patients before cART initiation might lead to enhanced cooperation with TB-specific CD4 T-cell responses. Although we have no data on specific anti-TB T-cell responses in the patients studied herein, HIV-specific T-cell responses do not seem to differ between IRIS and non-IRIS patients.22 It is therefore conceivable that higher NK cytolytic activity contributes to an increased Ag load (and to the induction of anti-TB T-cell responses) through M tuberculosis–infected cell lysis. Moreover, activated NK cells can induce cytokine secretion by monocytes, further enhancing CD8 T-cell responses to M tuberculosis.13 These phenomena could be amplified by effective TB therapy and by the immune restoration induced by cART.23 It is also possible that NK-cell and specific T-cell responses are amplified by a defective regulatory T-cell response, as reported by Seddki et al.24 Indeed, regulatory T cells have been shown to suppress immune responses, including NK-cell activation.25 Therefore, the interaction between innate and adaptive immune responses may increase the risk of IRIS by activating specific immune cells and enhancing the secretion of proinflammatory cytokines that, in turn, may sustain NK-cell activation.26 Increased CD4 T-cell production of proinflammatory cytokines such as IL6, IL8, TNF-α, and IFN-γ and elevated serum levels of these mediators have been reported in IRIS patients compared with non-IRIS patients.27 Although NK cells can produce large amounts of IFN-γ, the increased IFN-γ levels reported before and during IRIS22,28 may not be entirely because of NK cells, because we observed no difference between IRIS and non-IRIS patients in terms of IFN-γ production by NK cells after target cell stimulation, only a trend toward higher spontaneous IFN-γ secretion by NK cells from IRIS patients. Other immune cells, such as activated T cells, may be the main source of IFN-γ. Indeed, a recent study of Cambodian patients28 showed that TB-IRIS was associated with a slower increase in in vitro T-cell IFN-γ responses to RD1 TB Ags and PPD than in IRIS-free controls. The investigators concluded that other features of M tuberculosis–specific immune restoration are important in the pathogenesis of IRIS, but they did not study NK-cell responses.
The degranulation capacity of NK cells was similar in HIV+/TB+ patients at IRIS onset and in matched non-IRIS patients, unlike the situation before cART. The baseline difference in NK-cell degranulation activity between IRIS and non-IRIS patients was abrogated during cART by an increase in non-IRIS patients, possibly because of global immune reconstitution. Although NK cells studied at IRIS onset expressed similar levels of the CD69 activation marker, expression of NK cell–activating receptors (NKp30, NKp46, and NKG2D) was lower in IRIS patients. These results indicate that the mechanisms controlling the activity of NK cells are active just before or during IRIS, leading to down-regulation of these receptors. Possible explanations include the development of regulatory feedback mechanisms or the internalization of NK-cell receptors after ligand binding before or at the time of IRIS onset. Regulatory feedback at IRIS onset may be exerted either by regulatory T cells or by subsets of NK cells secreting anti-inflammatory cytokines such as IL-10 and TGF-β.29,30 IL10-producing NK cells could suppress specific T-cell responses,31 thereby controlling exaggerated responses during TB-IRIS.23 IL10 and TGF-β are also able to down-regulate NK cell–activating receptors.25,32 The role of the different subsets of NK cells in IRIS needs to be further explored.
Several studies have indicated a role for NK cells in HIV infection, but to our knowledge, this is the first study of NK cells in HIV/M tuberculosis–coinfected patients. Our results support the hypothesis that patients who develop IRIS during cART have higher innate responses to M tuberculosis–infected cells, as suggested by the elevated cytotoxic activity of their NK cells before cART initiation. In agreement with our hypothesis, higher baseline proportions of Vδ2+TCRγδ+ T cells lacking KIR expression were recently reported in IRIS patients compared with non-IRIS patients.23 Because Vγ9Vδ2 T cells are a major peripheral-blood γδT-cell subset displaying broad reactivity against TB Ags, these data, together with our present results, point to a more effective innate immune effector response against M tuberculosis Ags before cART initiation in patients who go on to develop IRIS.
The results of the present study suggest that a NK-cell degranulation assay performed before cART initiation could serve as a predictive marker of IRIS in TB+/HIV+ patients. Our results also highlight the contribution of NK cells to the pathogenesis of IRIS in cART-treated HIV-infected patients with TB.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all of the patients who participated in this study and the medical staff of Khmer Soviet Friendship Hospital in Phnom Penh, Dongkeo Referral Hospital in Takeo, Preah Kossamak Hospital in Phnom Penh, and Svay Rieng Referral Hospital.
This work was supported by the Agence Nationale de Recherche sur le Sida (ANRS; the French National Agency for AIDS and Hepatitis Research) project number 12153.
Authorship
Contribution: P.P and E.N. designed and performed the research, analyzed the data, and wrote the manuscript; Y.M., L.B., C.S., K.P., D.L., M.F., O.M., and S.T analyzed the data and wrote the manuscript; G.P., F.B.-S., and D.S.-A. designed the research, analyzed the data, and wrote the manuscript. A scientific committee sponsored by the ANRS designed this study and oversaw the statistical analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A list of the members of the CAMELIA study team and investigators is available in the online supplemental Appendix.
Correspondence: Daniel Scott-Algara, Institut Pasteur, Department of Virology, Unité de Régulation des Infections Rétrovirales, 25 rue du Docteur Roux, 75015 Paris, France; e-mail: daniel.scott-algara@pasteur.fr.

![Figure 3. Degranulation capacity and IFN-γ production by target-stimulated NK cells before cART initiation. Total degranulation (total CD107a+ expression [A]), degranulation without IFN-γ production (CD107a+IFN-γ− [B]), and total IFN-γ production (IFN-γ+ [C]) are compared across the different groups. For HIV-infected patients, the results are those obtained before cART. Results are medians (75th-25th percentiles). Outside values are indicated by points. Significant P values are indicated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/14/10.1182_blood-2011-09-377523/4/m_zh89991288810003.jpeg?Expires=1769096237&Signature=ZvVEdXYH0c3P8aizA3g1LXfQW3ULt2mbtop1QUnymUseDfSJORTstazEdb9Dg1qL2zfCelwnyz31Cmc19S9GpEWZlhF0Z6T~9HkfhT3LoQtb~9~Bh8RYjIp3cg9eRnBvfhhl7Q~blbYBRDgbmmPdCr5i8DYBSF93AduF7b86p-D9N-2~aqdJRjJnMhhKVQYVzC~YxJMYXc9lsaouyy6yhawPW66YI452hQh2tbrwubSfVAKppwwQp5jMZyE9VHYBkAR6NIfJ~NfbTpkhdwZptDl1J19U~-nnOv7wdy3ixtfXGjMPOc8FKwFB8TaS~x-MQuezz1mdYhHM1X1qlJKUfA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal