Abstract
Adult T-cell leukemia (ATL) and human T-cell lymphotropic virus type I (HTLV-I)–associated myelopathy/tropical spastic paraparesis (HAM/TSP) are known to be caused by HTLV-I infection. However, current methods used to determine HTLV-I infection do not differentiate between HTLV-I asymptomatic carriers (ACs) and ATL and HAM/TSP patients. Using the luciferase immunoprecipitation system, a highly sensitive, quantitative technology that can efficiently detect HTLV-I Ab responses, we examined Ab responses for HTLV-I in serum/plasma samples from 439 subjects in Jamaica, including HTLV-I–seronegative donors, ACs, and ATL and HAM/TSP patients. The Ab responses of HTLV-I–infected subjects differed significantly from those of seronegative donors for all 3 immunodominant proteins, Gag, Env, and Tax. HAM/TSP patients had significantly higher Ab responses for Gag and Env compared with ACs, and Ab responses for all 3 Ags were higher in HAM/TSP patients than in ATL patients. Moreover, immunoreactivities for HTLV-I Ags as determined by the luciferase immunoprecipitation system could distinguish HAM/TSP patients from ACs at a true-positive rate of 85.42% and from ATL patients at a true-positive rate of 75.00%, and modeled in conjunction with subject information to distinguish HAM/TSP patients from ACs (odds ratio = 14.12) and from ATL patients (odds ratio = 7.00). The relative risk assessment resulting from these significant differences between Ab responses in HTLV-I–infected groups may be a useful diagnostic tool in the future.
Introduction
Human T-cell lymphotropic virus type I (HTLV-I) was the first human retrovirus that was shown to be associated with an aggressive CD4+ T-cell malignancy called adult T-cell leukemia/lymphoma (ATL),1-3 and a chronic, progressive neurologic disorder called HTLV-I–associated myelopathy/tropical spastic paraparesis (HAM/TSP).4,5 HTLV-I has also been shown to be associated with several other inflammatory disorders, including uveitis, arthropathy, and polymyositis.6-8 Endemic regions for HTLV-I include Japan, the Caribbean, South America, and central regions of Africa and the Middle East.9 Whereas the majority of HTLV-I–infected individuals remain asymptomatic carriers (ACs), up to 5% of the approximate 20 million HTLV-I–infected people worldwide develop ATL or HAM/TSP.9 However, it remains unknown how the virus can lead to such different diseases and why only small numbers of HTLV-I–infected individuals develop these diseases. Therefore, it is of interest to explore biomarkers that may predict risks of developing an HTLV-I–related disease outcome relative to those HTLV-I–infected individuals who remain asymptomatic. Such a prognostic biomarker may consist of a single gene or molecule or may be the product of an algorithm of numerous indicators.10
Several HTLV-I–specific responses, including proviral DNA load, HTLV-I–specific T-cell response, and HTLV-I–specific Ab responses, have been associated with the pathogenesis of HTLV-I–related diseases.11,12 Whereas both the mean proviral load and Ab titer are elevated in ATL and HAM/TSP patients compared with ACs, ATL patients typically have a high mean HTLV-I proviral load and high Ab titer against only HTLV-I structural proteins, but lack Ab responses against Tax.13-15 In contrast, HTLV-I–Tax-specific Abs are elevated in HAM/TSP patients compared with both ACs and ATL patients.16,17 In addition, HTLV-I–specific T cells are activated in HAM/TSP patients, but not in ATL patients.18-21 Distinct virological and immunologic responses related to HTLV-I may result from a dysregulation of immune control against HTLV-I infection and may be associated with the process underlying ATL and HAM/TSP. However, the assays to evaluate virological and immunologic responses, such as PCR and flow cytometry, are cell-based assays and typically are not high throughput, and furthermore, no diagnostic tests can distinguish or predict the clinical outcome of an HTLV-I infection. As reported previously, Ab responses against HTLV-I are stable up to approximately 5 years after seroconversion of transfusion22 and mother-to-child transmission.23 HAM/TSP patients also had stable Ab responses for HTLV-I, but the group analysis showed higher Ab responses for HTLV-I compared with ACs,24 suggesting that Ab responses for HTLV-I may help in discriminating the clinical outcome of HTLV-I infection. Therefore, a simple, robust, and preferably serological test that can discriminate between ACs and ATL and HAM/TSP patients would have obvious clinical utility.
Most epidemiologic studies have relied on serological screening for Abs to HTLV-I using an enzyme immunoassay, with confirmatory testing by another method such as Western blot, immunofluorescence assay, or radioimmunoprecipitation assay.9 The PCR assay has been used for the detection of HTLV-I proviral DNA and for discriminatory typing between HTLV-I and HTLV-II. However, most immunoassays measuring anti–HTLV-I Abs do not quantitatively evaluate multiple Ags, and few assays produce a quantitative measure of Ab responses and are based on dilution comparisons. We recently described the luciferase immunoprecipitation system (LIPS), a highly sensitive, quantitative technology that can efficiently detect HTLV-I Ag-specific Ab responses in serum of HTLV-I–infected subjects.24 In addition, the LIPS assay can evaluate immunoreactivities for each HTLV-I Gag, Env, and Tax in HTLV-I–infected individuals, suggesting that HTLV-I–infected groups, ACs, and ATL and HAM/TSP patients might have differential LIPS immunoreactive patterns. In this present study, we have extended our preliminary analysis to detect anti–HTLV-I Abs in serum/plasma samples from 439 subjects in Jamaica, including HTLV-I–seronegative donors (NDs), ACs, and ATL and HAM/TSP patients. The Ab responses of HTLV-I–infected subjects differed significantly from those of NDs for all 3 immunodominant proteins, Gag, Env, and Tax. More specifically, HAM/TSP patients had higher level of Ab responses to all 3 immunodominant proteins compared with ATL patients, whereas ATL patients had significantly higher Ab responses for Env but lower for Tax compared with ACs. The Ab responses to HTLV-I Gag, Env, and Tax among the HTLV-I–infected groups (ACs, ATL, and HAM/TSP) were statistically modeled to classify these study groups. The classification model confirmed that HAM/TSP patients could be distinguished from ACs and ATL patients. These results demonstrated that profiling HTLV-I Ag-specific Ab responses in HTLV-I–infected subjects might be a useful quantitative diagnostic tool. Its use in predicting risk of disease in HTLV-I–infected individuals bears assessment in prospectively collected specimens that include pre- and postdiagnostic specimens.
Methods
Subjects
The subjects for the present analysis were participants in research studies conducted at the University of the West Indies (UWI), Kingston, Jamaica, in collaboration with the National Cancer Institute, National Institutes of Health (NIH; Bethesda, MD). All serum and/or plasma samples from study subjects were tested previously for HTLV by ELISA (Dupont) or enzyme immunoassay (Vironostika; Organo Teknika); seropositive samples were tested previously by Western blot (Cambridge Biotech or Genelabs Diagnostics HTLV-I blot 2.4). Serum/plasma samples obtained from a total of 439 subjects, including 167 HTLV-seronegative donors, 133 HTLV-I–seropositive ACs, 90 ATL patients, and 49 HAM/TSP patients, were the basis of the current analysis. The NDs were selected from participants in a nested case-control study.25 Of a total of 225 HTLV-I–seronegative donors (who had an HTLV-seronegative ELISA result or an HTLV-seropositive ELISA result and no bands on Western blot), 167 subjects were selected based on having > 1 vial of serum available from a NIH/Jamaican repository so that at least 1 sample remained in the repository from each subject. The ACs were also selected from subjects who participated in the nested case-control study of risk factors for HTLV-I seropositivity conducted among food handlers from the Kingston and Clarendon parishes of Jamaica in 1987-1988.25 Samples from these subjects were obtained from either that study, or a previous seroprevalence study that these subjects participated in between 1985-1986.26 Of a total of 201 subjects with HTLV-I–positive Western blot results, 133 subjects with > 1 vial of serum available to keep at least one sample in the repository from each subject were selected for the current analysis. Subjects with ATL were selected from among ATL patients identified through a nation-wide disease registry who were referred to the UWI clinic in 1984-2006.27 For the current analysis, we randomly selected 30 subjects from each of the 3 predominant ATL subtypes (acute, lymphoma, and chronic) for a total of 90 subjects. Subjects with HAM/TSP were selected among participants in an island-wide registry conducted in 1988-1998. From a total of 90 subjects, we selected 49 subjects who had an HTLV-I–positive Western blot and > 1 vial of serum or plasma available to keep at least 1 sample in the repository from each subject.28 The distributions of demographic factors for each group are summarized in Table 1. Informed consent was obtained from all participants in accordance with the Declaration of Helsinki. Study protocols followed the human experimentation guidelines of the US Department of Health and Human Services and Institutional Review Board approvals at the National Cancer Institute and UWI.
Distribution of demographic factors among study groups
| Factor . | NDs (n = 167) . | ACs (n = 133) . | ATL (n = 90) . | HAM/TSP (n = 49) . |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 31 (18.6) | 22 (16.5) | 41 (45.6) | 13 (26.5) |
| Female | 135 (80.8) | 111 (83.5) | 49 (54.4) | 36 (73.5) |
| Missing | 1 (0.6) | |||
| Race, n (%) | ||||
| African descent | 164 (98.2) | 132 (99.2) | 87 (96.7) | 42 (85.7) |
| Caucasian descent | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.0) |
| Other | 0 (0.0) | 1 (3.0) | 0 (0.0) | 2 (4.1) |
| Missing | 3 (1.8) | 3 (3.3) | 4 (8.2) | |
| Age, y* | ||||
| Mean ± SEM | 42.0 ± 1.1 | 43.1 ± 1.2 | 46.3 ± 1.7 | 48.3 ± 1.8 |
| Range | (18.3-78.0) | (18.0-75.9) | (18.7-80.7) | (14.8-75.0) |
| Factor . | NDs (n = 167) . | ACs (n = 133) . | ATL (n = 90) . | HAM/TSP (n = 49) . |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 31 (18.6) | 22 (16.5) | 41 (45.6) | 13 (26.5) |
| Female | 135 (80.8) | 111 (83.5) | 49 (54.4) | 36 (73.5) |
| Missing | 1 (0.6) | |||
| Race, n (%) | ||||
| African descent | 164 (98.2) | 132 (99.2) | 87 (96.7) | 42 (85.7) |
| Caucasian descent | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.0) |
| Other | 0 (0.0) | 1 (3.0) | 0 (0.0) | 2 (4.1) |
| Missing | 3 (1.8) | 3 (3.3) | 4 (8.2) | |
| Age, y* | ||||
| Mean ± SEM | 42.0 ± 1.1 | 43.1 ± 1.2 | 46.3 ± 1.7 | 48.3 ± 1.8 |
| Range | (18.3-78.0) | (18.0-75.9) | (18.7-80.7) | (14.8-75.0) |
Information on age was missing for 1 ND subject and 1 HAM/TSP subject.
Generation of Ruc-Ag fusion proteins
HTLV-I cDNA clones for Gag, Env, and Tax inserted into pREN2, a mammalian Renilla luciferase (Ruc) expression vector, were kindly provided by Dr P. Burbelo (National Institute of Dental and Craniofacial Research, NIH).24 Two micrograms of each mammalian expression vector with the HTLV-I gene was transfected into 293T cells in a 100-mm2 plate using FuGENE6 transfection reagent (Roche Diagnostics) and cultured for 48 hours in a 5% CO2 incubator at 37°C. The cultured cells were collected and lysed in buffer including 50mM Tris, pH 7.5, 100mM NaCl, 5mM MgCl2, 1% Triton X-100, 50% glycerol, and protease inhibitor cocktail tablets (Roche). The cell lysates were stored at −80°C until use. Light units (LUs) of each Ruc-Ag cell extract were measured in an LB 960 Centro microplate luminometer (Berthold Technologies) using coelenterazine substrate mix (Renilla Luciferase Assay System; Promega).
LIPS assay
The LIPS assay was performed in a 96-well plate format at room temperature as described previously.24 First, a “master plate” was constructed by diluting samples 1:10 in assay buffer A (20mM Tris, pH 7.5, 150mM NaCl, 5mM MgCl2, and 1% Triton X-100) in a 96-well polypropylene microtiter plate. For evaluating Ab titers by LIPS, 80 μL of buffer A, 20 μL of diluted samples, and 100 μL of the equivalent of 1 × 107 LUs of Ruc-Ag cell extract diluted in buffer A were added to each well of a polypropylene plate and incubated for 1 hour at room temperature on a rotary shaker. Next, 5 μL of a 30% suspension of Ultralink protein A/G beads (Thermo Scientific) in PBS were added to the bottom of each well of a 96-well filter HTS plate (Millipore), and 100 μL of Ag-Ab reaction mixture was transferred to the filter plate and incubated for 1 hour at room temperature on a rotary shaker. The plate was washed 8 times with buffer A, and then twice with PBS using the MultiScreenHTS Vacuum Manifold (Millipore). After the final washing, LUs were measured in an LB 960 Centro microplate luminometer (Berthold Technologies) using the Renilla Luciferase Assay reagent (Promega). All LU data were corrected for background by subtracting the LU values of beads incubated with Ruc-Ag cell extract but without sera and were obtained from the average of triplicate wells. All data from independent experiments were normalized using the LU values of positive control sera from a well-known HAM/TSP patient.
Statistical analysis
The Kruskal-Wallis test was used to compare ages among the 4 subject groups. The χ2 test and the Fisher exact test were used to compare the sex and racial distributions among the groups, respectively. The Wilcoxon 2-sample test was used to compare Ab levels against Gag, Env, and Tax between the ND group and the AC, ATL, and TSP groups; between the AC group and the ATL and TSP groups; between the ATL and TSP groups; and between each ATL subtype groups (ie, acute, chronic, and lymphoma).
Classification modeling
Three models were constructed among HTLV-I–infected subjects: HAM/TSP patients versus ACs, HAM/TSP patients versus ATL patients, and ATL patients versus ACs. For each model, generalized linear modeling was performed on log (base = 2)–transformed LIPS data available for each subject, in addition to sex, race, and age in the statistical software R (http://cran.r-project.org/) using the binomial error distribution in the less than 70%::30% cross-validation condition with replacement for a total of 30 rounds. For each round, model-assigned patient scores for the hold-out set were used to tally classification success iteratively using a criterion equal to the model-assigned score for each held-out subject. The median accuracy across all rounds was determined, and classification success across rounds was summarized via receiver operator curves. For within-round criterions providing accuracy equal to the optimal observed median accuracy across all rounds, the median was taken and defined as the classification criterion to use under the training condition. Modeling was then repeated under the training condition and the total success rate for the training model was determined using this criterion. Corresponding odds ratio (ORs) and P values for the resulting classifications were calculated using the method of Wald.
Results
Characteristics of the study population
The demographic characteristics of the study groups are summarized in Table 1. Mean ages of the study groups varied from 42 years in the ND group to 48 years in the HAM/TSP group (P = .01). The majority of each group was composed of female subjects, although the proportion of female subjects in each group ranged from 54% in the ATL group to 83% in the AC group (P < .0001). All study groups were predominantly of African descent (P = .10 based on comparison of African descent and Caucasian descent).
Ab responses for HTLV-I proteins in Jamaican subjects
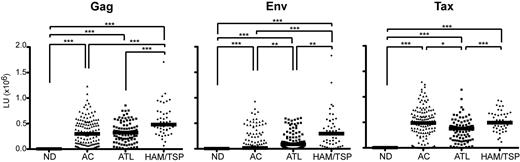
Ab responses for 3 immunodominant proteins, HTLV-I Gag, Env, and Tax, were analyzed in NDs, ACs, ATL patients, and HAM/TSP patients. The distributions of each Ab response are summarized by study group in Table 2. Compared with the ND group, the HTLV-I–infected groups had higher Ab responses against Gag, Env, and Tax. The median Ab response to Gag in the ND group was 0 LUs, compared with 299 750 LUs in ACs, 323 226 LUs in ATL patients, and 481 624 LUs in HAM/TSP patients. The median Ab response to Env was 0 LUs in NDs, compared with 16 665 LUs in ACs, 88 832 LUs in ATL patients, and 297 002 LUs in HAM/TSP patients. The median Ab response to Tax was 391 LUs in the ND group, compared with 486 826 LUs in ACs, 377 527 LUs in ATL patients, and 494 729 LUs in HAM/TSP patients (Table 2). Figure 1 shows the distribution of Ab responses by study group. Ab responses to all 3 Ags were significantly elevated in each of the HTLV-I–infected groups compared with those of the ND group (Figure 1; P < .0001 for all comparisons).
Ab responses against HTLV-I Ag in Jamaican cohorts
| HTLV-I Ab . | NDs (n = 167) . | ACs (n = 133) . | ATL (n = 90) . | HAM/TSP (n = 49) . |
|---|---|---|---|---|
| Anti-Gag | ||||
| Mean | 64 | 334591 | 330481 | 548383 |
| Median | 0 | 299750 | 323226 | 481624 |
| Range | (0-1353) | (0-1230011) | (64-855825) | (650635-1707570) |
| SD | 167 | 263166 | 205722 | 292195 |
| Anti-Env | ||||
| Mean | 96 | 141217 | 169071 | 352103 |
| Median | 0 | 16665 | 88832 | 297002 |
| Range | (0-1317) | (0-930648) | (0-604100) | (5223-1826905) |
| SD | 167 | 213800 | 191248 | 349616 |
| Anti-Tax | ||||
| Mean | 596 | 473064 | 348315 | 499228 |
| Median | 391 | 486826 | 377527 | 494729 |
| Range | (0-13582) | (0-1294444) | (232-1140863) | (279-930505) |
| SD | 1183 | 320642 | 217095 | 212571 |
| HTLV-I Ab . | NDs (n = 167) . | ACs (n = 133) . | ATL (n = 90) . | HAM/TSP (n = 49) . |
|---|---|---|---|---|
| Anti-Gag | ||||
| Mean | 64 | 334591 | 330481 | 548383 |
| Median | 0 | 299750 | 323226 | 481624 |
| Range | (0-1353) | (0-1230011) | (64-855825) | (650635-1707570) |
| SD | 167 | 263166 | 205722 | 292195 |
| Anti-Env | ||||
| Mean | 96 | 141217 | 169071 | 352103 |
| Median | 0 | 16665 | 88832 | 297002 |
| Range | (0-1317) | (0-930648) | (0-604100) | (5223-1826905) |
| SD | 167 | 213800 | 191248 | 349616 |
| Anti-Tax | ||||
| Mean | 596 | 473064 | 348315 | 499228 |
| Median | 391 | 486826 | 377527 | 494729 |
| Range | (0-13582) | (0-1294444) | (232-1140863) | (279-930505) |
| SD | 1183 | 320642 | 217095 | 212571 |
Ab responses against HTLV-I Gag, Env, and Tax from NDs, ACs, and ATL and HAM/TSP patients in Jamaica. The data were obtained from 167 NDs, 133 ACs, 90 ATL patients, and 49 HAM/TSP patients. Each HTLV-I–infected group showed significantly higher responses for HTLV-I Gag, Env, and Tax compared with NDs (P < .0001 for all 3 proteins). The horizontal line represents the median. *P < .01; **P < .001; ***P < .0001.
Ab responses against HTLV-I Gag, Env, and Tax from NDs, ACs, and ATL and HAM/TSP patients in Jamaica. The data were obtained from 167 NDs, 133 ACs, 90 ATL patients, and 49 HAM/TSP patients. Each HTLV-I–infected group showed significantly higher responses for HTLV-I Gag, Env, and Tax compared with NDs (P < .0001 for all 3 proteins). The horizontal line represents the median. *P < .01; **P < .001; ***P < .0001.
Each HTLV-I–infected group was profiled based on Ab responses for HTLV-I Gag, Env, and Tax. The AC group had significantly higher Ab responses to all 3 proteins compared with the ND group (Figure 1; P < .0001 in all 3 proteins). The AC group had a similar median Ab response to HTLV-I Gag to the ATL group (299 750 and 323 226 LUs, respectively; Table 2 and Figure 1), and a similar median anti-Tax Ab response to the HAM/TSP group (486 826 LUs and 494 729 LUs, respectively; Table 2 and Figure 1). However, the AC group had a lower median Ab response to HTLV-I Env (16 665 LUs) than ATL and HAM/TSP patients (88 832 and 297 002 LUs, respectively; Table 2 and Figure 1). Ab responses for HTLV-I Tax had a broader range of values in ACs than in ATL and HAM/TSP patients (Table 2 and Figure 1).
ATL and HAM/TSP patients also demonstrated differential Ab responses for HTLV-I Gag, Env, and Tax. Specifically, the median values of Ab responses to all 3 Ags were highly elevated in HAM/TSP patients compared with ATL patients (Figure 1; P < .0001 in Gag and Tax, P < .001 in Env). HAM/TSP patients had significantly higher Ab responses to Gag (median, 481 624 LUs) and Env (median, 297 002 LUs), but not to Tax, than the AC group (Figure 1; P < .0001 in Gag and Env). ATL patients were found to have a significantly higher median Ab response to Env (median, 88 832), but significantly lower Ab responses to Tax (median, 377 527) compared with the AC group (Figure 1; P < .001 for Env and P < .01 for Tax).
When a cutoff value for immunoreactivity was defined as a value that was 2 SDs above the mean value for the ND group (eg, anti-Gag, 398 LUs; anti-Env, 430 LUs; and anti-Tax, 2960 LUs), all HAM/TSP patients had immunoreactivity against both Gag and Env and there were only 2 HAM/TSP patients without anti-Tax immunoreactivity. In contrast, 16.5% of ACs and 7.8% of ATL patients lacked Ab responses to Gag, Env, or Tax. In the Jamaican cohort, HTLV-I–infected groups with or without HTLV-related diseases showed immunoreactivity against HTLV-I Gag, Env, and/or Tax. Specifically, HAM/TSP patients showed immunoreactivity against all 3 HTLV-I proteins compared with the more heterogenous responses in the AC group and in ATL patients.
Classification modeling
Given the differential patterns of Ab responses for HTLV-I Ags in HTLV-I–infected subjects, we explored how well models constructed on these responses in conjunction with sex, race, and age can distinguish HAM/TSP from ACs (Model 1), HAM/TSP from ATL (Model 2), and ATL from ACs (Model 3).
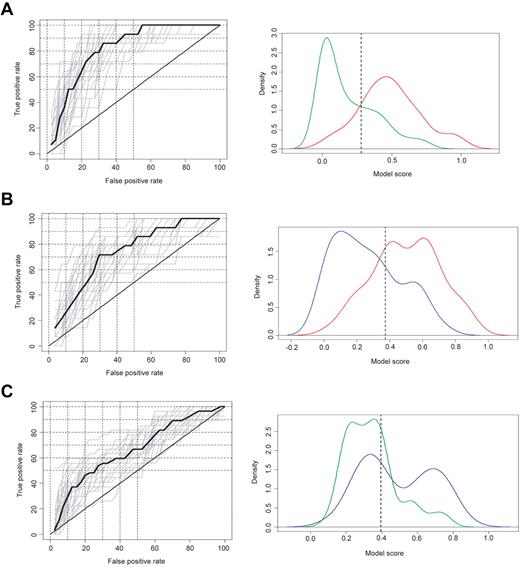
Under cross-validation modeling conditions, the Model 1 classification was better than that for Model 2 and Model 2 was better than that for Model 3. Specifically, Model 1 distinguished HAM/TSP patients from ACs at a median true-positive rate of 78.57% and a median false-positive rate of 27.50% (Figure 2A left graph). Model 2 distinguished HAM/TSP patients from ATL patients at a median true-positive rate of 71.43% and a median false-positive rate of 29.63% (Figure 2B left graph). Model 3 distinguished ATL from ACs at a median true-positive rate of 59.26% and a median false-positive rate of 40.00% (Figure 2C left graph).
Classification model: cross-validation image of Ab responses against HTLV-I. The HTLV-I–infected groups: Model 1, comparing HAM/TSP patients and ACs (A); Model 2, comparing HAM/TSP and ATL patients (B); and Model 3, comparing ATL patients and ACs (C) were modeled and classified in conjunction with sex, race, and age using the Ab responses for Gag, Env, and Tax. Left graphs show receiver operating characteristic curves with cross-validation in each model. Right graphs show a model score under training conditions. Green, red, and blue curves represent the model score of ACs, HAM/TSP patients, and ATL patients, respectively.
Classification model: cross-validation image of Ab responses against HTLV-I. The HTLV-I–infected groups: Model 1, comparing HAM/TSP patients and ACs (A); Model 2, comparing HAM/TSP and ATL patients (B); and Model 3, comparing ATL patients and ACs (C) were modeled and classified in conjunction with sex, race, and age using the Ab responses for Gag, Env, and Tax. Left graphs show receiver operating characteristic curves with cross-validation in each model. Right graphs show a model score under training conditions. Green, red, and blue curves represent the model score of ACs, HAM/TSP patients, and ATL patients, respectively.
Under training modeling conditions using classification criteria based on the cross-validation modeling condition, Model 1 classification remained better than that for Model 2 and Model 2 again was better than that for Model 3. Specifically, Model 1 distinguished HAM/TSP patients from ACs at a true-positive rate of 85.42% and a false-positive rate of 29.32% (Table 3 and Figure 2A right graph). Model 2 distinguished HAM/TSP patients from ATL patients at a true-positive rate of 75.00% and a false-positive rate of 30.00% (Table 3 and Figure 2B right graph). Model 3 distinguished ATL patients from ACs at a true-positive rate of 58.89% and a false-positive rate of 27.82% (Table 3 and Figure 2C right graph). Comparison of weights across these models revealed that Ab responses for HTLV-I Env and sex were significant contributors to each model (Table 4). Whereas Ab responses for HTLV-I Tax and race were significant contributors to Model 1 only and Ab responses for HTLV-I Gag were significant to Model 2 only (Table 4), age did not contribute significantly to any model (Table 4).
Performance of classification models
| Comparison Class 1 Class 2 . | Model 1: HAM/TSP ACs . | Model 2: HAM/TSP ATL . | Model 3: ATL ACs . |
|---|---|---|---|
| Classification criterion | 0.2814487 | 0.3755479 | 0.3961038 |
| Class 1 categorized to: | |||
| True-positive rate, % | 85.42 | 75.00 | 58.89 |
| False-negative rate, % | 14.58 | 25.00 | 41.11 |
| Class 2 categorized to: | |||
| True-negative rate, % | 29.32 | 30.00 | 27.82 |
| False-positive rate, % | 70.68 | 70.00 | 72.18 |
| Total success rate, % | 74.59 | 71.74 | 66.82 |
| Comparison Class 1 Class 2 . | Model 1: HAM/TSP ACs . | Model 2: HAM/TSP ATL . | Model 3: ATL ACs . |
|---|---|---|---|
| Classification criterion | 0.2814487 | 0.3755479 | 0.3961038 |
| Class 1 categorized to: | |||
| True-positive rate, % | 85.42 | 75.00 | 58.89 |
| False-negative rate, % | 14.58 | 25.00 | 41.11 |
| Class 2 categorized to: | |||
| True-negative rate, % | 29.32 | 30.00 | 27.82 |
| False-positive rate, % | 70.68 | 70.00 | 72.18 |
| Total success rate, % | 74.59 | 71.74 | 66.82 |
Coefficient of classification models
| Comparison Class 1 Class 2 . | Model 1: HAM/TSP ACs . | Model 2: HAM/TSP ATL . | Model 3: ATL ACs . |
|---|---|---|---|
| Intercept | −16.5958 (P = .000514) | −23.608039 (P = .000109) | −0.89417 (P = .762800) |
| LIPS reactivity | |||
| Gag | 0.50717 (P = .060680) | 0.812071 (P = .007285) | 0.03462 (P = .694000) |
| Env | 0.42052 (P = .000103) | 0.215104 (P = .033557) | 0.10923 (P = .028200) |
| Tax | −0.32341 (P = .011454) | −0.020447 (P = .883214) | −0.06642 (P = .313600) |
| Sex | −1.04694 (P = .033379) | −0.94039 (P = .034573) | −1.45376 (P = .000026) |
| Race | 3.17668 (P = .001173) | 1.345414 (P = .083781) | 0.72871 (P = .581500) |
| Age, y | 0.0154 (P = .313567) | 0.008538 (P = .561598) | 0.01115 (P = .288600) |
| Comparison Class 1 Class 2 . | Model 1: HAM/TSP ACs . | Model 2: HAM/TSP ATL . | Model 3: ATL ACs . |
|---|---|---|---|
| Intercept | −16.5958 (P = .000514) | −23.608039 (P = .000109) | −0.89417 (P = .762800) |
| LIPS reactivity | |||
| Gag | 0.50717 (P = .060680) | 0.812071 (P = .007285) | 0.03462 (P = .694000) |
| Env | 0.42052 (P = .000103) | 0.215104 (P = .033557) | 0.10923 (P = .028200) |
| Tax | −0.32341 (P = .011454) | −0.020447 (P = .883214) | −0.06642 (P = .313600) |
| Sex | −1.04694 (P = .033379) | −0.94039 (P = .034573) | −1.45376 (P = .000026) |
| Race | 3.17668 (P = .001173) | 1.345414 (P = .083781) | 0.72871 (P = .581500) |
| Age, y | 0.0154 (P = .313567) | 0.008538 (P = .561598) | 0.01115 (P = .288600) |
Bold values contributed significantly to the model.
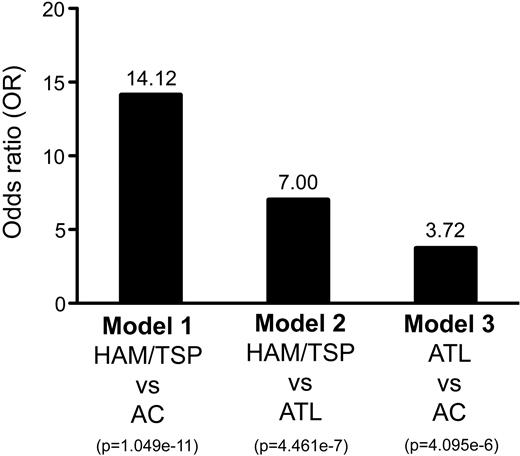
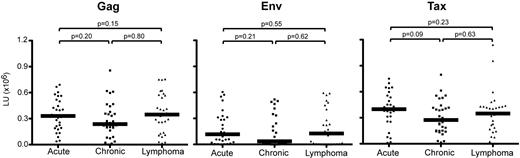
Our results provide evidence that immunoreactivities for HTLV-I Ags by LIPS can be modeled in conjunction with subject information to distinguish HAM/TSP patients from ACs (OR = 14.12, Figure 3), HAM/TSP patients from ATL patients (OR = 7.00, Figure 3), and ATL patients from ACs (OR = 3.72, Figure 3). However, it is important to emphasize that the ability to distinguish ATL patients from ACs was notably lower by comparison. On closer inspection of the distribution of model-assigned subject scores (Figure 2C right graph), a bimodal distribution of ATL patients is evident. These results suggest that ATL patients may include 2 subtypes, 1 more AC like and 1 less AC like. Figure 4 shows the distribution of Ab responses in ATL patients by ATL subtype: acute, chronic, or lymphoma. Inspection of Ab responses across ATL patients stratified by ATL subtype was consistently uniform (Figure 4). It is unclear why 41% of ATL patients could not be distinguished from ACs (Table 3).
Estimated OR of disease risk for ACs and ATL and HAM/TSP patients in Jamaica. The HTLV-I–infected groups: Model 1, comparing HAM/TSP patients and ACs; Model 2, comparing HAM/TSP and ATL patients; and Model 3, comparing ATL patients and ACs were modeled and classified in conjunction with sex, race, and age using the Ab responses for Gag, Env, and Tax. Corresponding OR and P values for the resulting classifications were calculated using the method of Wald.
Estimated OR of disease risk for ACs and ATL and HAM/TSP patients in Jamaica. The HTLV-I–infected groups: Model 1, comparing HAM/TSP patients and ACs; Model 2, comparing HAM/TSP and ATL patients; and Model 3, comparing ATL patients and ACs were modeled and classified in conjunction with sex, race, and age using the Ab responses for Gag, Env, and Tax. Corresponding OR and P values for the resulting classifications were calculated using the method of Wald.
Ab responses against HTLV-I Gag, Env, and Tax of ATL patients in Jamaica. Data were obtained from acute, chronic, and lymphoma ATL patients (n = 30 each). The horizontal line represents the median.
Ab responses against HTLV-I Gag, Env, and Tax of ATL patients in Jamaica. Data were obtained from acute, chronic, and lymphoma ATL patients (n = 30 each). The horizontal line represents the median.
Discussion
We reported previously that HTLV-I–infected subjects had immunoreactivities for HTLV-I Gag, Env, and Tax by LIPS.24 In the present study, we extended this analysis to detect anti–HTLV-I Abs in 439 subjects from Jamaica, where the seroprevalence of HTLV-I has been reported to be 6%, increases with age, and is universally higher among women than men.26 The results of the present study demonstrated a differential pattern of Ab responses for HTLV-I Gag, Env, and Tax between HTLV-I–infected and uninfected subjects, as well as between ACs and ATL and HAM/TSP patients. This differential Ab response enabled us to develop a classification model to discriminate HTLV-I–infected asymptomatic individuals from patients with HAM/TSP. The HTLV-I–infected groups, including ACs and ATL and HAM/TSP patients, showed significantly higher immunoreactivity for all 3 HTLV-I proteins compared with NDs. Specifically, HAM/TSP patients had significantly higher Ab responses for Gag and Env, but not for Tax, compared with ACs (Figure 1). Likewise, Ab responses for all 3 HTLV-I proteins were higher in HAM/TSP patients than in ATL patients (Figure 1). HAM/TSP is a systemic immune-mediated inflammatory disease characterized by high HTLV-I viral load, high cellular immune responses, and high humoral immune responses.16,17,19,29-32 Robust antiviral activities, especially against Tax, have been reported to mimic the level of viral replication and disease status in HAM/TSP patients.19,32-34 In the present study, Ab responses for HTLV-I Gag and for Env were significantly elevated in HAM/TSP patients. This suggests that both anti-Gag and anti-Env Abs may be associated with disease status and may be used as classification tools for HAM/TSP patients in HTLV-I–infected subjects. We also reported previously that HAM/TSP patients had high Ab responses for HTLV-I Ags by LIPS, in particular, immunoreactivities for HTLV-I Env were significantly elevated in HAM/TSP patients compared with ACs and ATL patients.24 Therefore, the present results were consistent with and strengthen previous reports that Ab responses for HTLV-I are highly elevated in HAM/TSP patients.
The 2 distinct pathologies associated with HTLV-I infection, ATL and HAM/TSP, have not been linked to any specific mutations within the virus.35 Epidemiologic studies using serological data and clinical records enable us to observe the spread of HTLV-I infection in different parts of the world and to determine the spectrum of associated diseases.15,36,37 There are various serological screening methods currently available to test for Abs to HTLV-I, including the enzyme immunoassay and Western blot.9 Candidate serum markers of immune activation and their association with HTLV-I infection and associated disease states have also been reported previously.38-41 However, most immunoassays measuring anti–HTLV-I Abs do not quantitatively evaluate multiple Ags and are incapable of detecting conformational epitopes within these Ags. In contrast, the LIPS assay used in this study provides quantitative measures of Ab responses for multiple virus proteins that can be used to efficiently evaluate humoral responses in patients and controls.42,43 Based on these quantitative measures of Ab responses for HTLV-I Gag, Env, and Tax by the LIPS assay, we modeled and classified the HTLV-I Ab responses in conjunction with subject information, sex, race, and age, between HAM/TSP patients and ACs (Model 1), between HAM/TSP patients and ATL patients (Model 2), and between ATL patients and ACs (Model 3). Under training modeling conditions using classification criteria based on cross-validation modeling conditions, Model 1 distinguished HAM/TSP patients from ACs at a true-positive rate of 85.42% and a false-positive rate of 29.32% (Table 3 and Figure 2A right graph). Model 2 distinguished HAM/TSP patients from ATL patients at a true-positive rate of 75.00% and a false-positive rate of 30.00% (Table 3 and Figure 2B right graph). Based on this algorithm, HAM/TSP patients are 14.12 times more likely to have a particular LIPS reactivity pattern than are ACs and 7.00 times more likely than are ATL patients (Figure 3). Because each HTLV-I–infected group (ACs and ATL and HAM/TSP patients) from Jamaica had a particular LIPS-reactive pattern, these classification models were able to distinguish HAM/TSP patients from ACs and ATL patients. Moreover, comparison of weights across these models revealed that Ab responses for HTLV-I Env and sex are significant contributors to each Model (Table 4). Ab responses for HTLV-I Tax and race are significant contributors to Model 1 only and Ab responses for HTLV-I Gag significant to Model 2 only (Table 4). Ab responses for HTLV-I Env have been reported to be a possible biomarker for HTLV-I–related disease onset.24,44,45 These results suggested that elevated Ab responses for HTLV-I Env might be a predictive marker for onset and type of HTLV-I–related diseases. Another coefficient of cross-validation, sex, also contributed in each model, but age did not. Female preponderances of HAM/TSP have been reported for endemic areas, but the associations of other factors were also suggested for the higher incidence rate of HAM/TSP in women than in men.28,46 Compared with Model 1 and Model 2, the ability to distinguish ATL patients from ACs (Model 3) was notably lower by comparison, although the OR for Model 3 was estimated at 3.72 (Figure 3). There did not appear to be a bias for ATL subtype (ie, acute, chronic, or lymphoma), but other information, such as disease duration, might affect Ab responses for HTLV-I. Longitudinal analysis of Ab responses for HTLV-I might provide strong evidence for distinguishing between ATL patients and ACs. Therefore, the results of the present study demonstrate the usefulness of serological and immunologic responses for HTLV-I for classification modeling of HTLV-I–related diseases. In addition to Ab responses for HTLV-I Ags, other serological markers such as neopterin, tryptophan, cytokines, and chemokines, which have been reported previously to be possible biomarkers of HTLV-I–related diseases,38-40 might provide additional evidence and less misclassification for further classification modeling of HTLV-I–related diseases by serological testing.
The LIPS assay is a simple, sensitive, and high-throughput technology to produce a quantitative measure of Ab responses for HTLV-I. The high throughput of the LIPS assay, combined with its ability to produce quantitative values of anti–HTLV-I Ab responses, demonstrates its potential as an extremely efficient tool for the clinical investigation of HTLV-I–related diseases. A previous study24 also demonstrated that HAM/TSP patients had higher Ab responses for HTLV-I Env compared with ACs and ATL patients, which is consistent with the results of the current study. However, differences in LIPS immunoreactivities observed for other HTLV-I Ags may be attributed to increased patient cohort size rather than to technical differences. Therefore, comparison of large cohorts from different endemic areas would be needed for the generalization of assay data across different studies. In addition, confirmation of our classification models is also needed in larger studies from different endemic regions. Because independent data are not only needed to bring confidence to the binary modeling results, classification modeling methods requiring large balanced numbers of samples can alternatively be used with additional data (eg, multinomial modeling). Our results should be interpreted with caution: because this study was based on a cross-sectional study design, the results represent associations between Ab response patterns and disease states and Ab response patterns cannot be interpreted as predictors of disease. Application of the LIPS assay to prospectively collected samples that include pre- and postdiagnostic samples from subjects who develop ATL or HAM/TSP are needed to assess the usefulness of this assay for predicting clinical disease risk among HTLV-I–infected subjects and for tracking disease progression.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr James Goedert (National Cancer Institute, NIH) for coordinating the specimens and subjects for this analysis; Ms Norma Kim (Research Triangle Institute, Rockville, MD) for identifying the subjects for this analysis and arranging for the selection of serum/plasma samples; Dr Barrie Hanchard and Ms Beverley Cranston (the University of the West Indies, Jamaica) for patient recruitment; and Dr Peter Burbelo (National Institute of Dental and Craniofacial Research, NIH) for kindly providing us with HTLV-I Gag, Env, and Tax/pRen2 plasmids.
This research was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke, NIH.
National Institutes of Health
Authorship
Contribution: Y.E.-A. performed most of the experimental work and wrote the manuscript; A.A. performed some of the experimental work and wrote the manuscript; K.R.J. performed the statistical analysis, contributed to discussions, and wrote the manuscript; E.M.M. identified the subjects for analysis, arranged for the selection of serum/plasma samples, contributed to discussions, and wrote the manuscript; and S.J. supervised the project, contributed to discussions, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steven Jacobson, PhD, Viral Immunology Section, Neuroimmunology Branch, National Institute of Neurological Disorders and Stroke, NIH, Bethesda, MD 20892; e-mail: jacobsons@ninds.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal